Abstract
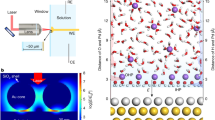
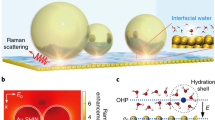
The dynamics and chemistry of interfacial water are essential components of electrocatalysis because the decomposition and formation of water molecules could dictate the protonation and deprotonation processes on the catalyst surface. However, it is notoriously difficult to probe interfacial water owing to its location between two condensed phases, as well as the presence of external bias potentials and electrochemically induced reaction intermediates. An atomically flat single-crystal surface could offer an attractive platform to resolve the internal structure of interfacial water if advanced characterization tools are developed. To this end, here we report a protocol based on the combination of in situ Raman spectroscopy and ab initio molecular dynamics (AIMD) simulations to unravel the directional molecular features of interfacial water. We present the procedures to prepare single-crystal electrodes, construct a Raman enhancement mode with shell-isolated nanoparticle, remove impurities, eliminate the perturbation from bulk water and dislodge the hydrogen bubbles during in situ electrochemical Raman experiments. The combination of the spectroscopic measurements with AIMD simulation results provides a roadmap to decipher the potential-dependent molecular orientation of water at the interface. We have prepared a detailed guideline for the application of combined in situ Raman and AIMD techniques; this procedure may take a few minutes to several days to generate results and is applicable to a variety of disciplines ranging from surface science to energy storage to biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
Data generated or analyzed during this study are included in this article and ref. 44. Source data are provided with this paper.
Code availability
The code that supports the findings of this research is available from the corresponding authors upon reasonable request.
References
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Kendrick, E., Kendrick, J., Knight, K. S., Islam, M. S. & Slater, P. R. Cooperative mechanisms of fast-ion conduction in gallium-based oxides with tetrahedral moieties. Nat. Mater. 6, 871–875 (2007).
Lim, H. et al. A universal approach for the synthesis of mesoporous gold, palladium and platinum films for applications in electrocatalysis. Nat. Protoc. 15, 2980–3008 (2020).
Wang, X. S., Xu, C. C., Jaroniec, M., Zheng, Y. & Qiao, S. Z. Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions. Nat. Commun. 10, 1–8 (2019).
Mubeen, S. et al. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 8, 247–251 (2013).
Ye, K., Wang, G. X. & Bao, X. H. Electrodeposited Sn-based catalysts for CO2 electroreduction. Chin. J. Struc. Chem. 39, 206–213 (2020).
Velasco-Velez, J. J. et al. The structure of interfacial water on gold electrodes studied by x-ray absorption spectroscopy. Science 346, 831–834 (2014).
Li, F. et al. Interplay of electrochemical and electrical effects induces structural transformations in electrocatalysts. Nat. Catal. 4, 479–487 (2021).
Wang, H. et al. Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nat. Commun. 6, 7261 (2015).
Subbaraman, R. et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012).
Zhang, B. K. et al. Insights into the H2O/V2O5 interface structure for optimizing water-splitting. Chin. J. Struc. Chem. 39, 189–199 (2020).
Mesa, C. A. et al. Multihole water oxidation catalysis on haematite photoanodes revealed by operando spectroelectrochemistry and DFT. Nat. Chem. 12, 82–89 (2020).
Wang, T. et al. Enhancing oxygen reduction electrocatalysis by tuning interfacial hydrogen bonds. Nat. Catal. 4, 753–762 (2021).
Tian, X. et al. Engineering bunched Pt–Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 366, 850–856 (2019).
Li, F. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 577, 509–513 (2020).
Li, K. et al. Enhancement of lithium-mediated ammonia synthesis by addition of oxygen. Science 374, 1593–1597 (2021).
Garcia de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Qing, G. et al. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 120, 5437–5516 (2020).
Vidal-Iglesias, F. J., Solla-Gullon, J., Herrero, E., Aldaz, A. & Feliu, J. M. Pd adatom decorated (100) preferentially oriented Pt nanoparticles for formic acid electrooxidation. Angew. Chem. Int. Ed. 49, 6998–7001 (2010).
Hines, M. A. & Zare, R. N. The interaction of Co with Ni(111)—rainbows and rotational trapping. J. Chem. Phys. 98, 9134–9147 (1993).
Marcandalli, G., Villalba, M. & Koper, M. T. M. The importance of acid–base equilibria in bicarbonate electrolytes for CO2 electrochemical reduction and CO reoxidation studied on Au (hkl) electrodes. Langmuir 37, 5707–5716 (2021).
Ledezma-Yanez, I. et al. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2, 17031 (2017).
Zhu, S. Q., Qin, X. P., Yao, Y. & Shao, M. H. pH-dependent hydrogen and water binding energies on platinum surfaces as directly probed through surface-enhanced infrared absorption spectroscopy. J. Am. Chem. Soc. 142, 8748–8754 (2020).
Dunwell, M., Yan, Y. & Xu, B. A surface-enhanced infrared absorption spectroscopic study of pH dependent water adsorption on Au. Surf. Sci. 650, 51–56 (2016).
Montenegro, A. et al. Asymmetric response of interfacial water to applied electric fields. Nature 594, 62–65 (2021).
Tong, Y., Lapointe, F., Thamer, M., Wolf, M. & Campen, R. K. Hydrophobic water probed experimentally at the gold electrode/aqueous interface. Angew. Chem. Int. Ed. 56, 4211–4214 (2017).
Liu, W. T. & Shen, Y. R. In situ sum-frequency vibrational spectroscopy of electrochemical interfaces with surface plasmon resonance. Proc. Natl Acad. Sci. USA 111, 1293–1297 (2014).
Ye, Y. et al. Using soft x-ray absorption spectroscopy to characterize electrode/electrolyte interfaces in-situ and operando. J. Electron. Spectrosc. Relat. Phenom. 221, 2–9 (2017).
Nong, H. N. et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 587, 408–413 (2020).
Blasco-Ahicart, M., Soriano-Lopez, J., Carbo, J. J., Poblet, J. M. & Galan-Mascaros, J. R. Polyoxometalate electrocatalysts based on earth-abundant metals for efficient water oxidation in acidic media. Nat. Chem. 10, 24–30 (2018).
Nie, S. & Emory, S. R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275, 1102–1106 (1997).
Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 57, 783–826 (1985).
Liu, X. L. et al. Filter-based ultralow-frequency Raman measurement down to 2 cm−1 for fast Brillouin spectroscopy measurement. Rev. Sci. Instrum. 88, 053110 (2017).
Liang, L. et al. Low-frequency shear and layer-breathing modes in Raman scattering of two-dimensional materials. ACS Nano. 11, 11777–11802 (2017).
Fleischmann, M., Hendra, P. J., Hill, I. R. & Pemble, M. E. Enhanced Raman spectra from species formed by the coadsorption of halide ions and water molecules on silver electrodes. J. Electroanal. Chem. 117, 243–255 (1981).
Funtikov, A. M., Sigalaev, S. K. & Kazarinov, V. E. Surface enhanced Raman scattering and local photoemission currents on the freshly prepared surface of a silver electrode. J. Electroanal. Chem. 228, 197–218 (1987).
Chen, Y. X. & Tian, Z. Q. Dependence of surface enhanced Raman scattering of water on the hydrogen evolution reaction. Chem. Phys. Lett. 281, 379–383 (1997).
Zou, S. Z., Chen, Y. X., Mao, B. W., Ren, B. & Tian, Z. Q. SERS studies on electrode/electrolyte interfacial water I. Ion effects in the negative potential region. J. Electroanal. Chem. 424, 19–24 (1997).
Chen, Y. X., Zou, S. Z., Huang, K. Q. & Tian, Z. Q. SERS studies of electrode/electrolyte interfacial water part II—librations of water correlated to hydrogen evolution reaction. J. Raman Spectrosc. 29, 749–756 (1998).
Jiang, Y. X. et al. Characterization of surface water on Au core Pt-group metal shell nanoparticles coated electrodes by surface-enhanced Raman spectroscopy. Chem. Commun. 28, 4608–4610 (2007).
Li, J. F. et al. SERS and DFT study of water on metal cathodes of silver, gold and platinum nanoparticles. Phys. Chem. Chem. Phys. 12, 2493–2502 (2010).
Li, J. F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010).
Li, C. Y. et al. In situ probing electrified interfacial water structures at atomically flat surfaces. Nat. Mater. 18, 697–701 (2019).
Wang, Y. H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Pham, T. A., Ping, Y. & Galli, G. Modelling heterogeneous interfaces for solar water splitting. Nat. Mater. 16, 401–408 (2017).
Selcuk, S. & Selloni, A. Facet-dependent trapping and dynamics of excess electrons at anatase TiO2 surfaces and aqueous interfaces. Nat. Mater. 15, 1107–1112 (2016).
Tuckerman, M., Laasonen, K., Sprik, M. & Parrinello, M. Ab initio molecular dynamics simulation of the solvation and transport of hydronium and hydroxyl ions in water. J. Chem. Phys. 103, 150–161 (1995).
Pham, T. A., Lee, D., Schwegler, E. & Galli, G. Interfacial effects on the band edges of functionalized si surfaces in liquid water. J. Am. Chem. Soc. 136, 17071–17077 (2014).
Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 241, 20–22 (1973).
Wu, L., Cao, D., Huang, Y. & Li, B. G. Poly(l-lactic acid)/SiO2 nanocomposites via in situ melt polycondensation of l-lactic acid in the presence of acidic silica sol: preparation and characterization. Polymer 49, 742–748 (2008).
Alekseeva, M. V. et al. NiCuMo-SiO2 catalyst for pyrolysis oil upgrading: model acidic treatment study. Appl. Catal. A 573, 1–12 (2019).
Pekarek, R. T. et al. Intrinsic chemical reactivity of solid-electrolyte interphase components in silicon–lithium alloy anode batteries probed by FTIR spectroscopy. J. Mater. Chem. A 8, 7897–7906 (2020).
Cai, W. B. et al. Investigation of surface-enhanced Raman scattering from platinum electrodes using a confocal Raman microscope: dependence of surface roughening pretreatment. Surf. Sci. 406, 9–22 (1998).
Le Ru, E. C., Blackie, E., Meyer, M. & Etchegoin, P. G. Surface enhanced Raman scattering enhancement factors: a comprehensive study. J. Phys. Chem. C. 111, 13794–13803 (2007).
Lin, H. X. et al. Uniform gold spherical particles for single-particle surface-enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 15, 4130–4135 (2013).
Baltruschat, H., Rach, E. & Heitbaum, J. Correlation of SERS intensity potential profiles with adsorption/desorption peaks of pyridine on Au. J. Electroanal. Chem. 194, 109–122 (1985).
Li, J. F., Rudnev, A., Fu, Y. C., Bodappa, N. & Wandlowski, T. In situ SHINERS at electrochemical single-crystal electrode/electrolyte interfaces: tuning preparation strategies and selected applications. ACS Nano 7, 8940–8952 (2013).
Dong, J. C. et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermediates at platinum single-crystal surfaces. Nat. Energy 4, 60–67 (2019).
Zhang, W., Bas, A. D., Ghali, E. & Choi, Y. Passive behavior of gold in sulfuric acid medium. T. Nonferr. Metal. Soc. 25, 2037–2046 (2015).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Le, J., Iannuzzi, M., Cuesta, A. & Cheng, J. Determining potentials of zero charge of metal electrodes versus the standard hydrogen electrode from density-functional-theory-based molecular dynamics. Phys. Rev. Lett. 119, 016801 (2017).
Cheng, J., Liu, X., VandeVondele, J., Sulpizi, M. & Sprik, M. Redox potentials and acidity constants from density functional theory based molecular dynamics. Acc. Chem. Res. 47, 3522–3529 (2014).
Cheng, J. & VandeVondele, J. Calculation of electrochemical energy levels in water using the random phase approximation and a double hybrid functional. Phys. Rev. Lett. 116, 086402 (2016).
Cheng, J., Sulpizi, M. & Sprik, M. Redox potentials and pKa for benzoquinone from density functional theory based molecular dynamics. J. Chem. Phys. 131, 154504 (2009).
Costanzo, F., Sulpizi, M., Della Valle, R. G. & Sprik, M. The oxidation of tyrosine and tryptophan studied by a molecular dynamics normal hydrogen electrode. J. Chem. Phys. 134, 244508 (2011).
Ding, S. Y. et al. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 1, 16021 (2016).
Ding, S. Y., You, E. M., Tian, Z. Q. & Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 46, 4042–4076 (2017).
Li, J. F., Zhang, Y. J., Ding, S. Y., Panneerselvam, R. & Tian, Z. Q. Core-shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 117, 5002–5069 (2017).
Chernyshova, I. V., Somasundaran, P. & Ponnurangam, S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl Acad. Sci. USA 115, E9261–E9270 (2018).
Laibinis, P. E. et al. Comparison of the structures and wetting properties of self-assembled monolayers of n-alkanethiols on the coinage metal surfaces, copper, silver, and gold. J. Am. Chem. Soc. 113, 7152–7167 (2002).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2019YFA0705400), the National Natural Science Foundation of China (21925404, 21991151, 21902137, 22104124, 22109003 and 22021001), the Shenzhen Fundamental Research Program (no. GXWD20201231165807007-20200807111854001), the Soft Science Research Project of Guangdong Province (2017B030301013), the ‘111’ Project (B17027) and the State Key Laboratory of Fine Chemicals, Dalian University of Technology (KF2002). We thank the Major Science and Technology Infrastructure Project of Material Genome Big-science Facilities Platform supported by Municipal Development and Reform Commission of Shenzhen.
Author information
Authors and Affiliations
Contributions
J.-F.L., Z.-Q.T. and F.P. designed the project. Y.-H.W. and S.L. conceived of and designed the protocol. Y.-H.W., Y.-J.Z. and R.-Y.Z. performed the experiments and analyzed the results. S.L., Z.-L.Y. and S.Z. performed the computations and the data analysis. Y.-H.W., J.-C.D., Y.-J.Z. and S.L. wrote the protocol. All authors discussed the results and contributed to the manuscript review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Alexis Grimaud and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Wang, Y. H. et al. Nature 600, 81–85 (2021): https://doi.org/10.1038/s41586-021-04068-z
Li, C. Y. et al. Nat. Mater. 18, 697–701 (2019): https://doi.org/10.1038/s41563-019-0356-x
Supplementary information
Source data
Source Data Fig. 3
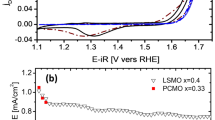
CV data normalized by electrode area.
Source Data Fig. 4
Fig. 4b,c,e, background normalized Raman data; Fig. 4d, unprocessed Raman data.
Source Data Fig. 5
Recorded Raman intensity.
Source Data Fig. 10
Normalized Raman data.
Source Data Fig. 11
Fig.11a, background normalized Raman data; Fig. 11b, unprocessed Raman data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, YH., Li, S., Zhou, RY. et al. In situ electrochemical Raman spectroscopy and ab initio molecular dynamics study of interfacial water on a single-crystal surface. Nat Protoc 18, 883–901 (2023). https://doi.org/10.1038/s41596-022-00782-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00782-8
This article is cited by
-
Hydrogen spillover bridged dual nano-islands triggered by built-in electric field for efficient and robust alkaline hydrogen evolution at ampere-level current density
Nano Research (2024)
-
CoIn dual-atom catalyst for hydrogen peroxide production via oxygen reduction reaction in acid
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.