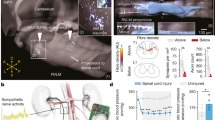
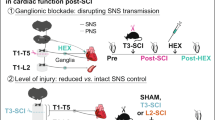
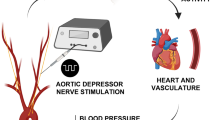
Abstract
Neurological disorders, including spinal cord injury, result in hemodynamic instability due to the disruption of supraspinal projections to the sympathetic circuits located in the spinal cord. We recently developed a preclinical model that allows the identification of the topology and dynamics through which sympathetic circuits modulate hemodynamics, supporting the development of a neuroprosthetic baroreflex that precisely controls blood pressure in rats, monkeys and humans with spinal cord injuries. Here, we describe the continuous monitoring of arterial blood pressure and sympathetic nerve activity over several months in preclinical models of chronic neurological disorders using commercially available telemetry technologies, as well as optogenetic and neuronal tract-tracing procedures specifically adapted to the sympathetic circuitry. Using a blueprint to construct a negative-pressure chamber, the approach enables the reproduction, in rats, of well-controlled and reproducible episodes of hypotension-mimicking orthostatic challenges already used in humans. Blood pressure variations can thus be directly induced and linked to the molecular, functional and anatomical properties of specific neurons in the brainstem, spinal cord and ganglia. Each procedure can be completed in under 2 h, while the construction of the negative-pressure chamber requires up to 1 week. With training, individuals with a basic understanding of cardiovascular physiology, engineering or neuroscience can collect longitudinal recordings of hemodynamics and sympathetic nerve activity over several months.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The datasets discussed in this protocol are available from Zenodo: https://doi.org/10.5281/zenodo.5227224.
Code availability
Aima is available from GitHub at https://github.com/neurorestore/Aima and as Supplementary Software 1.
References
McCorry, L. K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 71, 78 (2007).
Ruggiero, D. A., Cravo, S. L., Arango, V. & Reis, D. J. Central control of the circulation by the rostral ventrolateral reticular nucleus: anatomical substrates. Prog. Brain Res. 81, 49–79 (1989).
Kalia, M., Fuxe, K. & Goldstein, M. Rat medulla oblongata. III. Adrenergic (C1 and C2) neurons, nerve fibers and presumptive terminal processes. J. Comp. Neurol. 233, 333–349 (1985).
Schreihofer, A. M. & Guyenet, P. G. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J. Comp. Neurol. 387, 524–536 (1997).
Haselton, J. R. & Guyenet, P. G. Electrophysiological characterization of putative C1 adrenergic neurons in the rat. Neuroscience 30, 199–214 (1989).
Card, J. P. et al. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: implications for the central control of cardiovascular regulation. J. Comp. Neurol. 499, 840–859 (2006).
Squair, J. W. et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 590, 308–314 (2021).
Furlan, J. C., Fehlings, M. G., Shannon, P., Norenberg, M. D. & Krassioukov, A. V. Descending vasomotor pathways in humans: correlation between axonal preservation and cardiovascular dysfunction after spinal cord injury. J. Neurotrauma 20, 1351–1363 (2003).
Phillips, A. A. & Krassioukov, A. V. Contemporary cardiovascular concerns after spinal cord injury: mechanisms, maladaptations, and management. J. Neurotrauma 32, 1927–1942 (2015).
Halliday, G. M. et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann. Neurol. 27, 373–385 (1990).
Gai, W. P., Geffen, L. B., Denoroy, L. & Blessing, W. W. Loss of C1 and C3 epinephrine-synthesizing neurons in the medulla oblongata in Parkinson’s disease. Ann. Neurol. 33, 357–367 (1993).
Benarroch, E. E., Smithson, I. L., Low, P. A. & Parisi, J. E. Depletion of catecholaminergic neurons of the rostral ventrolateral medulla in multiple systems atrophy with autonomic failure. Ann. Neurol. 43, 156–163 (1998).
Illman, A., Stiller, K. & Williams, M. The prevalence of orthostatic hypotension during physiotherapy treatment in patients with an acute spinal cord injury. Spinal Cord. 38, 741–747 (2000).
Carlozzi, N. E. et al. Impact of blood pressure dysregulation on health-related quality of life in persons with spinal cord injury: development of a conceptual model. Arch. Phys. Med. Rehabil. 94, 1721–1730 (2013).
Wu, J.-C. et al. Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology 78, 1051–1057 (2012).
Cragg, J. J., Noonan, V. K., Krassioukov, A. & Borisoff, J. Cardiovascular disease and spinal cord injury: results from a national population health survey. Neurology 81, 723–728 (2013).
Popok, D. W., West, C. R., Hubli, M., Currie, K. D. & Krassioukov, A. V. Characterizing the severity of autonomic cardiovascular dysfunction after spinal cord injury using a novel 24 hour ambulatory blood pressure analysis software. J. Neurotrauma 34, 559–566 (2017).
Claassen, D. O., Adler, C. H., Hewitt, L. A. & Gibbons, C. Characterization of the symptoms of neurogenic orthostatic hypotension and their impact from a survey of patients and caregivers. BMC Neurol. 18, 125 (2018).
Watanabe, H. et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 125, 1070–1083 (2002).
Figueroa, J. J. et al. Multiple system atrophy: prognostic indicators of survival. Mov. Disord. 29, 1151–1157 (2014).
O’Sullivan, S. S. et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131, 1362–1372 (2008).
Asboth, L. et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci. 21, 576–588 (2018).
van den Brand, R. et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336, 1182–1185 (2012).
Takeoka, A., Vollenweider, I., Courtine, G. & Arber, S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 159, 1626–1639 (2014).
Grimm, D. et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol. 82, 5887–5911 (2008).
Witten, I. B. et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733 (2011).
Ueno, M., Ueno-Nakamura, Y., Niehaus, J., Popovich, P. G. & Yoshida, Y. Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat. Neurosci. 19, 784–787 (2016).
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Wenger, N. et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat. Med. 22, 138–145 (2016).
Wagner, F. B. et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71 (2018).
Capogrosso, M. et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539, 284–288 (2016).
Formento, E. et al. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 21, 1728–1741 (2018).
Capogrosso, M. et al. Configuration of electrical spinal cord stimulation through real-time processing of gait kinematics. Nat. Protoc. 13, 2031–2061 (2018).
Squair, J. W. et al. High thoracic contusion model for the investigation of cardiovascular function after spinal cord injury. J. Neurotrauma https://doi.org/10.1089/neu.2016.4518 (2016).
Claydon, V. E. & Krassioukov, A. V. Orthostatic hypotension and autonomic pathways after spinal cord injury. J. Neurotrauma 23, 1713–1725 (2006).
Krassioukov, A., Eng, J. J., Warburton, D. E. & Teasell, R., Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of the management of orthostatic hypotension after spinal cord injury. Arch. Phys. Med. Rehabil. 90, 876–885 (2009).
Courtine, G. et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 12, 1333–1342 (2009).
Brennan, F. H. et al. Acute post-injury blockade of α2δ-1 calcium channel subunits prevents pathological autonomic plasticity after spinal cord injury. Cell Rep. 34, 108667 (2021).
Mueller, P. J., Mischel, N. A. & Scislo, T. J. Differential activation of adrenal, renal, and lumbar sympathetic nerves following stimulation of the rostral ventrolateral medulla of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1230–R1240 (2011).
Mueller, P. J., Cunningham, J. T., Patel, K. P. & Hasser, E. M. Proposed role of the paraventricular nucleus in cardiovascular deconditioning. Acta Physiol. Scand. 177, 27–35 (2003).
Mueller, P. J., Foley, C. M., Vogl, H. W., Hay, M. & Hasser, E. M. Cardiovascular response to a group III mGluR agonist in NTS requires NMDA receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R198–R208 (2005).
Mueller, P. J. Exercise training attenuates increases in lumbar sympathetic nerve activity produced by stimulation of the rostral ventrolateral medulla. J. Appl. Physiol. 102, 803–813 (2007).
Pacher, P., Nagayama, T., Mukhopadhyay, P., Bátkai, S. & Kass, D. A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 3, 1422–1434 (2008).
Poormasjedi-Meibod, M.-S. et al. Experimental spinal cord injury causes left-ventricular atrophy and is associated with an upregulation of proteolytic pathways. J. Neurotrauma 36, 950–961 (2019).
DeVeau, K. M. et al. A comparison of passive hindlimb cycling and active upper-limb exercise provides new insights into systolic dysfunction after spinal cord injury. Am. J. Physiol. Heart Circ. Physiol. 313, H861–H870 (2017).
Squair, J. W. et al. Spinal cord injury causes systolic dysfunction and cardiomyocyte atrophy. J. Neurotrauma 35, 424–434 (2018).
Stocker, S. D. & Muntzel, M. S. Recording sympathetic nerve activity chronically in rats: surgery techniques, assessment of nerve activity, and quantification. Am. J. Physiol. Heart Circ. Physiol. 305, H1407–H1416 (2013).
West, C. R., Popok, D., Crawford, M. A. & Krassioukov, A. V. Characterizing the temporal development of cardiovascular dysfunction in response to spinal cord injury. J. Neurotrauma 32, 922–930 (2015).
Nout, Y. S., Beattie, M. S. & Bresnahan, J. C. Severity of locomotor and cardiovascular derangements after experimental high-thoracic spinal cord injury is anesthesia dependent in rats. J. Neurotrauma 29, 990–999 (2012).
Zhang, Y. et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J. Neurosci. 33, 12970–12981 (2013).
Rabchevsky, A. G. et al. Effects of gabapentin on muscle spasticity and both induced as well as spontaneous autonomic dysreflexia after complete spinal cord injury. Front. Physiol. 3, 329 (2012).
Yoshimoto, M., Miki, K., Fink, G. D., King, A. & Osborn, J. W. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension 55, 644–651 (2010).
Yoshimoto, M. et al. Renal and lumbar sympathetic nerve activity during development of hypertension in dahl salt-sensitive rats. Hypertension 74, 888–895 (2019).
Miki, K., Kosho, A. & Hayashida, Y. Method for continuous measurements of renal sympathetic nerve activity and cardiovascular function during exercise in rats. Exp. Physiol. 87, 33–39 (2002).
Miki, K., Oda, M., Kamijyo, N., Kawahara, K. & Yoshimoto, M. Lumbar sympathetic nerve activity and hindquarter blood flow during REM sleep in rats. J. Physiol. 557, 261–271 (2004).
Miki, K., Yoshimoto, M. & Tanimizu, M. Acute shifts of baroreflex control of renal sympathetic nerve activity induced by treadmill exercise in rats. J. Physiol. 548, 313–322 (2003).
Martel, E. et al. Mechanisms of the cardiovascular deconditioning induced by tail suspension in the rat. Am. J. Physiol. 274, H1667–H1673 (1998).
Tarasova, O., Figourina, I., Zotov, A., Borovik, A. & Vinogradova, O. Effect of tail suspension on haemodynamics in intact and sympathectomized rats. Eur. J. Appl. Physiol. 85, 397–404 (2001).
Louisy, F., Tran, C. C., Resch, G. & Luce, P. The venous tone is not altered after three-week tail suspension in rats. J. Gravit. Physiol. 5, 47–48 (1998).
Brizzee, B. L. & Walker, B. R. Altered baroreflex function after tail suspension in the conscious rat. J. Appl. Physiol. 69, 2091–2096 (1990).
Collins, J.-A., Rudenski, A., Gibson, J., Howard, L. & O’Driscoll, R. Relating oxygen partial pressure, saturation and content: the haemoglobin-oxygen dissociation curve. Breathe 11, 194–201 (2015).
Hatcher, J. D., Chiu, L. K. & Jennings, D. B. Anemia as a stimulus to aortic and carotid chemoreceptors in the cat. J. Appl. Physiol. 44, 696–702 (1978).
Santiago, T. V., Edelman, N. H. & Fishman, A. P. The effect of anemia on the ventilatory response to transient and steady-state hypoxia. J. Clin. Invest. 55, 410–418 (1975).
Sundlöf, G. & Wallin, B. G. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J. Physiol. 278, 525–532 (1978).
Scherrer, U., Vissing, S. F. & Victor, R. G. Effects of lower-body negative pressure on sympathetic nerve responses to static exercise in humans. Microneurographic evidence against cardiac baroreflex modulation of the exercise pressor reflex. Circulation 78, 49–59 (1988).
Phillips, A. A., Bredin, S. S. D., Cote, A. T., Drury, C. T. & Warburton, D. E. R. Aortic distensibility is reduced during intense lower body negative pressure and is related to low frequency power of systolic blood pressure. Eur. J. Appl. Physiol. 113, 785–792 (2013).
Cheriyan, T. et al. Spinal cord injury models: a review. Spinal Cord. 52, 588–595 (2014).
Demmin, G. L., Clase, A. C., Randall, J. A., Enquist, L. W. & Banfield, B. W. Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection. J. Virol. 75, 10856–10869 (2001).
Smith, B. N. et al. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc. Natl Acad. Sci. USA 97, 9264–9269 (2000).
Banfield, B. W., Kaufman, J. D., Randall, J. A. & Pickard, G. E. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J. Virol. 77, 10106–10112 (2003).
Hilton, B. J., Blanquie, O., Tedeschi, A. & Bradke, F. High-resolution 3D imaging and analysis of axon regeneration in unsectioned spinal cord with or without tissue clearing. Nat. Protoc. 14, 1235–1260 (2019).
Hart, E. C. et al. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am. J. Physiol. Heart Circ. Physiol. 312, H1031–H1051 (2017).
Acknowledgements
The present work was supported by a Consolidator Grant from the European Research Council (ERC-2015-CoG HOW2WALKAGAIN 682999), the Swiss National Science Foundation (subsides 310030_130850, CRSII5_183519, BSCGI0 1578000), Compute Canada, the Natural Sciences and Engineering Research Council, the Canadian Institutes of Health Research, Banting Fellowship, Alberta Innovates Health Solutions, Campus Alberta Neuroscience, the Libin Cardiovascular Institute, the Hotchkiss Brain Institute, PRAXIS, International Foundation for Research in Paraplegia, McCaig Institute for Bone and Joint Health. J.W.S. is supported by a CIHR Banting postdoctoral fellowship and a Marie Skłodowska-Curie individual fellowship (no. 842578). J.E.S. is supported by a CIHR Canada Graduate Scholarships - Doctoral Program scholarship, a BRAIN CREATE graduate scholarship, an Eyes High doctoral recruitment scholarship, and a Branch Out Neurological Foundation scholarship. We are grateful to Bernard Schneider and Theofanis Karayannis for providing viral vectors; Laura Batti and Ivana Gantar from the Advanced Lightsheet Imaging Center (ALICe) at the Wyss Center for Bio and Neuroengineering, Geneva; and Fabien Moreillon from Hepia, Campus Biotech Geneva.
Author information
Authors and Affiliations
Contributions
J.W.S., J.E.S., R.H. and L.M. designed and developed the surgical procedures. J.W.S., S.A. and M.G. and A.A.P. designed and developed the assessment equipment and procedures. J.W.S. and M.G. designed and developed Aima. J.W.S., V.P.P. and A.A.P. designed the lower-body negative-pressure chamber. Q.B. developed the histological and imaging procedures. A.A.P. and G.C. supervised the work. J.E.S., R.H., J.W.S., Q.B., A.A.P. and G.C. wrote the manuscript. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
G.C., A.A.P. and J.W.S. have filed several patents in relation to the present work. G.C. and A.A.P. are consultants of ONWARD medical. G.C. and A.A.P are shareholders of ONWARD, a company with direct relationships with the presented work.
Peer review
Peer review information
Nature Protocols thanks Alexander Rabchevsky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Squair, J. W. et al. Nature 590, 308–314 (2021): https://doi.org/10.1038/s41586-020-03180-w
Asboth, L. et al. Nat. Neurosci. 21, 576–588 (2018): https://doi.org/10.1038/s41593-018-0093-5
Supplementary information
Supplementary Note
Code blocks to reproduce each procedure in the protocol.
Supplementary Software 1
Library package for data analysis.
Source data
Source Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soriano, J.E., Hudelle, R., Squair, J.W. et al. Longitudinal interrogation of sympathetic neural circuits and hemodynamics in preclinical models. Nat Protoc 18, 340–373 (2023). https://doi.org/10.1038/s41596-022-00764-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00764-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.