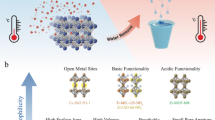
Abstract
Metal–organic frameworks (MOFs) are excellent candidates for water harvesting from desert air. MOF-303 (Al(OH)(PZDC), where PZDC is 1-H-pyrazole-3,5-dicarboxylate), a robust and water-stable MOF, is a particularly promising water-harvesting sorbent that can take up water at low relative humidity and release it under mild heating. Accordingly, development of a facile, high-yield synthesis method for its production at scale is highly desirable. Here we report detailed protocols for the green, water-based preparation of MOF-303 on both gram and kilogram scales. Specifically, four synthetic methods (solvothermal, reflux, vessel and microwave), involving different equipment requirements, are presented to guarantee general accessibility. Typically, the solvothermal method takes ~24 h to synthesize MOF-303, while the reflux and vessel methods can reduce the time to 4–8 h. With the microwave-assisted method, the reaction time can be further reduced to just 5 min. In addition, we provide guidance on the characterization of MOF-303, as well as water-harvesting MOFs in general, to ensure high quality of the product in terms of its purity, crystallinity, porosity and water uptake. Furthermore, to address the need for future commercialization of this material, we demonstrate that our protocol can be employed to produce 3.5 kg per batch with a yield of 91%. MOF-303 synthesized at this large scale shows similar crystallinity and water uptake capacity compared to the respective material produced at a small scale. Our synthetic procedure is green and water-based, and can produce the MOF within hours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
Data availability
The raw data for water vapor sorption and nitrogen sorption are available at https://doi.org/10.6084/m9.figshare.19859002.v3.
References
Liu, C.-H., Nguyen, H. L. & Yaghi, O. M. Harvesting water from desert air. AsiaChem 1, 18–25 (2020).
Mekonnen, M. M. & Hoekstra, A. Y. Four billion people facing severe water scarcity. Sci. Adv. 2, e1500323 (2016).
UN World Water Development Report: Nature-based Solutions for Water (UN, 2018); https://www.unwater.org/publications/world-water-development-report-2018/
Shiklomanov, I. A. World freshwater resources. Water in crisis: a guide to the world’s fresh water resources. Clim. Change 45, 13–24 (1993).
Al-Karaghouli, A. & Kazmerski, L. L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sust. Energ. Rev. 24, 343–356 (2013).
Bagheri, F. Performance investigation of atmospheric water harvesting systems. Water Resour. Ind. 20, 23–28 (2018).
Gido, B., Friedler, E. & Broday, D. M. Assessment of atmospheric moisture harvesting by direct cooling. Atmos. Res. 182, 156–162 (2016).
Hanikel, N. et al. Rapid cycling and exceptional yield in a metal–organic framework water harvester. ACS Cent. Sci. 5, 1699–1706 (2019).
Kim, H. et al. Adsorption-based atmospheric water harvesting device for arid climates. Nat. Commun. 9, 1191 (2018).
Kim, H. et al. Water harvesting from air with metal–organic frameworks powered by natural sunlight. Science 356, 430–434 (2017).
Xu, W. & Yaghi, O. M. Metal–organic frameworks for water harvesting from air, anywhere, anytime. ACS Cent. Sci. 6, 1348–1354 (2020).
Hanikel, N. et al. Evolution of water structures in metal–organic frameworks for improved atmospheric water harvesting. Science 374, 454–459 (2021).
Bagi, S., Wright, A. M., Oppenheim, J., Dincă, M. & Román-Leshkov, Y. Accelerated synthesis of a Ni2Cl2 (BTDD) metal–organic framework in a continuous flow reactor for atmospheric water capture. ACS Sustain. Chem. Eng. 9, 3996–4003 (2021).
Hanikel, N., Prévot, M. S. & Yaghi, O. M. MOF water harvesters. Nat. Nanotechnol. 15, 348–355 (2020).
Yang, P., Clark, D. S. & Yaghi, O. M. Envisioning the “air economy”—powered by reticular chemistry and sunlight for clean air, clean energy, and clean water. Mol. Front. J. 5, 30–37 (2021).
Burtch, N. C., Jasuja, H. & Walton, K. S. Water stability and adsorption in metal–organic frameworks. Chem. Rev. 114, 10575–10612 (2014).
Stock, N. & Biswas, S. Synthesis of metal–organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem. Rev. 112, 933–969 (2012).
Abtab, S. M. T. et al. Reticular chemistry in action: a hydrolytically stable MOF capturing twice its weight in adsorbed water. Chem 4, 94–105 (2018).
Ko, N. et al. Tailoring the water adsorption properties of MIL-101 metal–organic frameworks by partial functionalization. J. Mater. Chem. A 3, 2057–2064 (2015).
Rieth, A. J. et al. Record-setting sorbents for reversible water uptake by systematic anion exchanges in metal–organic frameworks. J. Am. Chem. Soc. 141, 13858–13866 (2019).
Rieth, A. J., Yang, S., Wang, E. N. & Dincă, M. Record atmospheric fresh water capture and heat transfer with a material operating at the water uptake reversibility limit. ACS Cent. Sci. 3, 668–672 (2017).
Wright, A. M., Rieth, A. J., Yang, S., Wang, E. N. & Dincă, M. Precise control of pore hydrophilicity enabled by post-synthetic cation exchange in metal–organic frameworks. Chem. Sci. 9, 3856–3859 (2018).
Fathieh, F. et al. Practical water production from desert air. Sci. Adv. 4, eaat3198 (2018).
Lenzen, D. et al. Scalable green synthesis and full‐scale test of the metal–organic framework CAU‐10‐H for use in adsorption‐driven chillers. Adv. Mater. 30, 1705869 (2018).
Rubio‐Martinez, M. et al. Scalability of continuous flow production of metal–organic frameworks. ChemSusChem 9, 938–941 (2016).
Tannert, N., Jansen, C., Nießing, S. & Janiak, C. Robust synthesis routes and porosity of the Al-based metal–organic frameworks Al-fumarate, CAU-10-H and MIL-160. Dalton Trans. 48, 2967–2976 (2019).
O’Keeffe, M., Peskov, M. A., Ramsden, S. J. & Yaghi, O. M. The reticular chemistry structure resource (RCSR) database of, and symbols for, crystal nets. Acc. Chem. Res. 41, 1782–1789 (2008).
Gropp, C. et al. Standard practices of reticular chemistry. ACS Cent. Sci. 6, 1255–1273 (2020).
Zhao, T. et al. High-yield, fluoride-free and large-scale synthesis of MIL-101 (Cr). Dalton Trans. 44, 16791–16801 (2015).
Permyakova, A. et al. Synthesis optimization, shaping, and heat reallocation evaluation of the hydrophilic metal–organic framework MIL‐160 (Al). ChemSusChem 10, 1419–1426 (2017).
Lenzen, D. et al. A metal–organic framework for efficient water-based ultra-low-temperature-driven cooling. Nat. Commun. 10, 3025 (2019).
Cong, S. et al. Highly water-permeable metal–organic framework MOF-303 membranes for desalination. J. Am. Chem. Soc. 143, 20055–20058 (2021).
Cho, K. H. et al. Rational design of a robust aluminum metal–organic framework for multi-purpose water-sorption-driven heat allocations. Nat. Commun. 11, 5112 (2020).
Kong, Y.-R. et al. Microwave-assisted rapid synthesis of nanoscale MOF-303 for hydrogel composites with superior proton conduction at ambient-humidity conditions. ACS Appl. Energy Mater. 4, 14681–14688 (2021).
Acknowledgements
We acknowledge financial support from Defense Advanced Research Projects Agency (DARPA) under contract HR0011-21-C-0020. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of DARPA. Helpful comments and suggestions on this work were provided by S. Cohen (DARPA) and D. Moore (GE). We thank E. Neumann from the Yaghi Group for useful suggestions regarding this manuscript. We acknowledge the College of Chemistry Nuclear Magnetic Resonance Facility for resource instruments, which are partially supported by NIH S10OD024998, and staff assistance from H. Celik and A. Lund. Z. Zheng thanks X. Han from the Yaghi Research Group for assisting in setting up the reactor. N.H. is thankful for financial support through a Kavli ENSI Philomathia Graduate Student Fellowship and a Blavatnik Innovation Fellowship.
Author information
Authors and Affiliations
Contributions
Z. Zheng, H.L.N., N.H. and O.M.Y. designed the experiments; Z. Zheng and N.H. performed the syntheses of MOF-303; Z. Zheng and H.L.N. designed the purification of MOF-303 and analyzed the collected data with guidance from O.M.Y.; Z. Zheng, H.L.N., K.K.-Y.L. and Z. Zhou performed the purification of kilogram-scale MOF-303. N.H. collected the water vapor isotherms of MOF-303 samples and interpreted the data; T.M. recorded SEM images for MOF-303 samples. All authors wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
O.M.Y. is co-founder of Water Harvesting Inc., aiming at commercializing related technologies.
Peer review
Peer review information
Nature Protocols thanks Ruzhu Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Hanikel, N. et al. ACS Cent. Sci. 5, 1699–1706 (2019): https://doi.org/10.1021/acscentsci.9b00745
Hanikel, N. et al. Science 374, 454–459 (2021): https://doi.org/10.1126/science.abj0890
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, Z., Nguyen, H.L., Hanikel, N. et al. High-yield, green and scalable methods for producing MOF-303 for water harvesting from desert air. Nat Protoc 18, 136–156 (2023). https://doi.org/10.1038/s41596-022-00756-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00756-w
This article is cited by
-
Sustainable moisture energy
Nature Reviews Materials (2024)
-
Continuous synthesis of boron-doped carbon nitride supported silver nanoparticles in an ultrasound-assisted coiled flow inverter microreactor
Journal of Flow Chemistry (2024)
-
A general large-scale synthesis approach for crystalline porous materials
Nature Communications (2023)
-
Biomimetic surface engineering for sustainable water harvesting systems
Nature Water (2023)
-
MOF water harvester produces water from Death Valley desert air in ambient sunlight
Nature Water (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.