Abstract
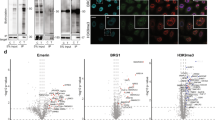
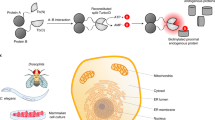
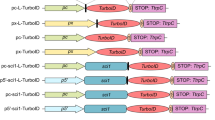
Proximity biotinylation is a commonly used method to identify the in vivo proximal proteome for proteins of interest. This technology typically relies on fusing a bait protein to a biotin ligase using overexpression or clustered regularly interspaced short palindromic repeats (CRISPR)-based tagging, thus prohibiting the use of such assays in cell types that are difficult to transfect or transduce. We recently developed an ‘off-the-shelf’ proximity biotinylation method that makes use of a recombinant enzyme consisting of the biotin ligase TurboID fused to the antibody-recognizing moiety Protein A. In this method, a bait-specific antibody and the ProteinA-Turbo enzyme are consecutively added to permeabilized fixed or unfixed cells. Following incubation, during which ProteinA-Turbo antibody–antigen complexes are formed, unbound molecules are washed away, after which bait-proximal biotinylation is triggered by the addition of exogenous biotin. Finally, biotinylated proteins are enriched from crude lysates using streptavidin beads followed by mass spectrometry-based protein identification. In principle, any scientist can perform this protocol within 3 days, although generating the proteomics data requires access to a high-end liquid chromatography–mass spectrometry setup. Data analysis and data visualization are relatively straightforward and can be performed using any type of software that converts raw mass spectrometry spectra files into identified and quantified proteins. The protocol has been optimized for nuclear targets but may also be adapted to other subcellular regions of interest.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE27 partner repository with the dataset identifier PXD030963, and processed data are provided in Supplementary Table 1. Source data are provided with this paper.
References
Richards, A. L., Eckhardt, M. & Krogan, N. J. Mass spectrometry‐based protein–protein interaction networks for the study of human diseases. Mol. Syst. Biol. 17, 1–18 (2021).
Smits, A. H. & Vermeulen, M. Characterizing protein–protein interactions using mass spectrometry: challenges and opportunities. Trends Biotechnol. 34, 825–834 (2016).
Roux, K. J., Kim, D. I., Raida, M. & Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 196, 801–810 (2012).
Samavarchi-Tehrani, P., Samson, R. & Gingras, A.-C. Proximity dependent biotinylation: key enzymes and adaptation to proteomics approaches. Mol. Cell. Proteom. 19, 757–773 (2020).
Zhao, X. et al. ultraID: a compact and efficient enzyme for proximity-dependent biotinylation in living cells. bioRxiv 2021.06.16.448656 (2021).
Kido, K. et al. AirID, a novel proximity biotinylation enzyme, for analysis of protein–protein interactions. Elife 9, 1–24 (2020).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. https://doi.org/10.1038/nbt.4201 (2018).
Go, C. D. et al. A proximity-dependent biotinylation map of a human cell. Nature 595, 120–124 (2021).
Rhee, H.-W. et al. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 339, 1328–1331 (2013).
Han, S., Li, J. & Ting, A. Y. Proximity labeling: spatially resolved proteomic mapping for neurobiology. Curr. Opin. Neurobiol. 50, 17–23 (2018).
Moquin, D. M. et al. Localized protein biotinylation at dna damage sites identifies zpet, a repressor of homologous recombination. Genes Dev. 33, 253 (2019).
van Mierlo, G. & Vermeulen, M. Chromatin proteomics to study epigenetics – challenges and opportunities. Mol. Cell. Proteom. 20, 100056 (2021).
Santos-Barriopedro, I., van Mierlo, G. & Vermeulen, M. Off-the-shelf proximity biotinylation for interaction proteomics. Nat. Commun. 12, 5015 (2021).
Bar, D. Z. et al. Biotinylation by antibody recognition - a method for proximity labeling. Nat. Methods 15, 127–133 (2018).
Li, X. et al. Defining proximity proteomics of histone modified proteins by antibody-mediated protein A-APEX2 labeling. Genom. Proteom. Bioinform. https://doi.org/10.1016/j.gpb.2021.09.003 (2022).
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 (2007).
St-Germain, J. R. et al. Variability in streptavidin-sepharose matrix quality can significantly affect proximity-dependent biotinylation (BioID) data. J. Proteome Res. 19, 3554–3561 (2020).
Villaseñor, R. et al. ChromID identifies the protein interactome at chromatin marks. Nat. Biotechnol. 38, 728–736 (2020).
Zhang, X. et al. Proteome-wide identification of ubiquitin interactions using UbIA-MS. Nat. Protoc. 13, 530–550 (2018).
Jeyaprakash, A. A. et al. Structure of a survivin-borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell 131, 271–285 (2007).
Przewloka, M. R. et al. CENP-C is a structural platform for kinetochore assembly. Curr. Biol. 21, 399–405 (2011).
Petrovic, A. et al. Structure of the MIS12 complex and molecular basis of its interaction with CENP-C at human kinetochores. Cell 167, 1028–1040 (2016).
Hayashi, T. et al. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell 118, 715–729 (2004).
McAinsh, A. D. & Meraldi, P. The CCAN complex: linking centromere specification to control of kinetochore-microtubule dynamics. Semin. Cell Dev. Biol. 22, 946–952 (2011).
Hadders, M. A. & Lens, S. M. A. Changing places: chromosomal passenger complex relocation in early anaphase. Trends Cell Biol. 32, 165–176 (2022).
Saksouk, N. et al. Redundant mechanisms to form silent chromatin at pericentromeric regions rely on BEND3 and DNA methylation. Mol. Cell 56, 580–594 (2014).
Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019).
Acknowledgements
We thank members of the Vermeulen lab for input during the development of the off-the-shelf proximity labeling approach. The Vermeulen lab is part of the Oncode Institute, which is partly funded by the Dutch Cancer Society. This work is further supported by an ERC consolidator grant to M.V. (771059). I.S.B. is supported by a Marie Sklodowska-Curie postdoctoral fellowship under the European Union’s Horizon 2020 research and innovation program (grant no. 835908). G.v.M. is supported by an EMBO long-term fellowship (no. 895-2020).
Author information
Authors and Affiliations
Contributions
I.S.B. and G.v.M. contributed equally to this work. I.S.B. and G.v.M. performed experiments and data analyses. I.S.B., G.v.M. and M.V. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
I.S.B., G.v.M. and M.V. have filed a patent related to this work (PCT/NL2022/050197).
Peer review
Peer review information
Nature Protocols thanks Dalia Barsyte-Lovejoy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Santos-Barriopedro, I. et al. Nat. Commun. 12, 5015 (2021): https://doi.org/10.1038/s41467-021-25338-4
Supplementary information
Supplementary Table 1
Processed data related to MS measurements described in Figs. 2 and 3
Source data
Source Data Fig. 1
Unprocessed western blots
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos-Barriopedro, I., van Mierlo, G. & Vermeulen, M. Off-the-shelf proximity biotinylation using ProtA-TurboID. Nat Protoc 18, 36–57 (2023). https://doi.org/10.1038/s41596-022-00748-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00748-w
This article is cited by
-
The development of proximity labeling technology and its applications in mammals, plants, and microorganisms
Cell Communication and Signaling (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.