Abstract
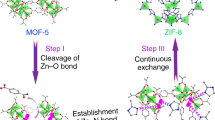
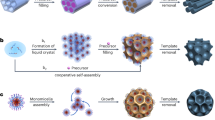
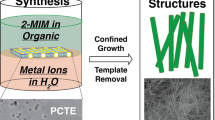
Metal-organic frameworks (MOFs), or porous coordination polymers, are crystalline porous materials formed by coordination bonding between inorganic and organic species on the basis of the self-assembly of the reacting units. The typical characteristics of MOFs, including their large specific surface areas, ultrahigh porosities and excellent thermal and chemical stabilities, as well as their great potential for chemical and structural modifications, make them excellent candidates for versatile applications. Their poor electrical conductivity, however, has meant that they have not been useful for electrochemical applications. Fortuitously, the direct carbonization of MOFs results in a rearrangement of the carbon atoms of the organic units into a network of carbon atoms, which means that the products have useful levels of conductivity. The direct carbonization of zeolitic imidazolate framework (ZIF)-type MOFs, particularly ZIF-8, has successfully widened the scope of possible applications of MOFs to include electrochemical reactions that could be used in, for example, energy storage, energy conversion, electrochemical biosensors and capacitive deionization of saline water. Here, we present the first detailed protocols for synthesizing high-quality ZIF-8 and its modified forms of hollow ZIF-8, core-shell ZIF-8@ZIF-67 and ZIF-8@mesostuctured polydopamine. Typically, ZIF-8 synthesis takes 27 h to complete, and subsequent nanoarchitecturing procedures leading to hollow ZIF-8, ZIF-8@ZIF-67 and ZIF-8@mPDA take 6, 14 and 30 h, respectively. The direct-carbonization procedure takes 12 h. The resulting nanoporous carbons are suitable for electrochemical applications, in particular as materials for supercapacitors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research papers42,48,50,52. Datasets of Fig. 6d and g have been made openly accessible at Figshare (https://doi.org/10.6084/m9.figshare.18515858.v1). Source data are provided with this paper.
References
Ahmad, T. & Zhang, D. A critical review of comparative global historical energy consumption and future demand: the story told so far. Energy Rep. 6, 1973–1991 (2020).
Omer, A. M. Energy use and environmental impacts: a general review. J. Renew. Sustain. Energy 1, 053101 (2009).
Iqbal, S., Khatoon, H., Hussain Pandit, A. & Ahmad, S. Recent development of carbon based materials for energy storage devices. Mater. Sci. Energy Technol. 2, 417–428 (2019).
Titirici, M.-M. et al. Sustainable carbon materials. Chem. Soc. Rev. 44, 250–290 (2015).
Sun, M.-H. et al. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 45, 3479–3563 (2016).
Brownson, D. A. C., Kampouris, D. K. & Banks, C. E. An overview of graphene in energy production and storage applications. J. Power Sources 196, 4873–4885 (2011).
Ye, M., Zhang, Z., Zhao, Y. & Qu, L. Graphene platforms for smart energy generation and storage. Joule 2, 245–268 (2018).
Wang, L. & Hu, X. Recent advances in porous carbon materials for electrochemical energy storage. Chem. Asian J. 13, 1518–1529 (2018).
Zhang, Q., Huang, J.-Q., Qian, W.-Z., Zhang, Y.-Y. & Wei, F. The road for nanomaterials industry: a review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small 9, 1237–1265 (2013).
Randviir, E. P., Brownson, D. A. C. & Banks, C. E. A decade of graphene research: production, applications and outlook. Mater. Today 17, 426–432 (2014).
Li, X. & Zhi, L. Graphene hybridization for energy storage applications. Chem. Soc. Rev. 47, 3189–3216 (2018).
Kairi, M. I. et al. Toward high production of graphene flakes—a review on recent developments in their synthesis methods and scalability. J. Mater. Chem. A 6, 15010–15026 (2018).
Li, W., Liu, J. & Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 1, 16023 (2016).
Zhai, Y. et al. Carbon materials for chemical capacitive energy storage. Adv. Mater. 23, 4828–4850 (2011).
Wu, L., Li, Y., Fu, Z. & Su, B.-L. Hierarchically structured porous materials: synthesis strategies and applications in energy storage. Natl Sci. Rev. 7, 1667–1701 (2020).
Borchardt, L. et al. Toward a molecular design of porous carbon materials. Mater. Today 20, 592–610 (2017).
Kudernac, T., Lei, S., Elemans, J. A. A. W. & De Feyter, S. Two-dimensional supramolecular self-assembly: nanoporous networks on surfaces. Chem. Soc. Rev. 38, 402–421 (2009).
Zhu, Y.-P., Ren, T.-Z., Ma, T.-Y. & Yuan, Z.-Y. Hierarchical structures from inorganic nanocrystal self-assembly for photoenergy utilization. Int. J. Photoenergy 2014, 498540 (2014).
Ariga, K. et al. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 20, 51–95 (2019).
Wang, Q. & Astruc, D. State of the art and prospects in metal-organic framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 120, 1438–1511 (2020).
Geng, K. et al. Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 16, 8814–8933 (2020).
Stergar, J. & Maver, U. Review of aerogel-based materials in biomedical applications. J. Solgel Sci. Technol. 77, 738–752 (2016).
Xia, W. et al. High-performance energy storage and conversion materials derived from a single metal-organic framework/graphene aerogel composite. Nano Lett. 17, 2788–2795 (2017).
Xuan, X. et al. Carbon nanomaterials from metal-organic frameworks: a new material horizon for CO2 reduction. Front. Chem. 8, 573797 (2020).
Yaghi, O. M., Li, G. & Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 378, 703–706 (1995).
Yaghi, O. M. & Li, H. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J. Am. Chem. Soc. 117, 10401–10402 (1995).
Li, H., Eddaoudi, M., O’Keeffe, M. & Yaghi, O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402, 276–279 (1999).
Zhang, X. et al. A historical overview of the activation and porosity of metal–organic frameworks. Chem. Soc. Rev. 49, 7406–7427 (2020).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013).
Park, K. S. et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl Acad. Sci. USA 103, 10186–10191 (2006).
Liu, B., Shioyama, H., Akita, T. & Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 130, 5390–5391 (2008).
Radhakrishnan, L. et al. Preparation of microporous carbon fibers through carbonization of Al-based porous coordination polymer (Al-PCP) with furfuryl alcohol. Chem. Mater. 23, 1225–1231 (2011).
Hu, M. et al. Direct carbonization of Al-based porous coordination polymer for synthesis of nanoporous carbon. J. Am. Chem. Soc. 134, 2864–2867 (2012).
Chaikittisilp, W. et al. Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes. Chem. Commun. 48, 7259–7261 (2012).
Jiang, H.-L. et al. From metal-organic framework to nanoporous carbon: toward a very high surface area and hydrogen uptake. J. Am. Chem. Soc. 133, 11854–11857 (2011).
Yang, S. J. et al. MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity. Chem. Mater. 24, 464–470 (2012).
Wang, C. et al. New strategies for novel MOF-derived carbon materials based on nanoarchitectures. Chem 6, 19–40 (2020).
Marpaung, F. et al. Metal–organic framework (MOF)-derived nanoporous carbon materials. Chem. Asian J. 14, 1331–1343 (2019).
Chaikittisilp, W., Ariga, K. & Yamauchi, Y. A new family of carbon materials: synthesis of MOF-derived nanoporous carbons and their promising applications. J. Mater. Chem. A 1, 14–19 (2013).
Wan, X. et al. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2, 259–268 (2019).
Kim, M. et al. Tailored nanoarchitecturing of microporous ZIF-8 to hierarchically porous double-shell carbons and their intrinsic electrochemical property. ACS Appl. Mater. Interfaces 12, 34065–34073 (2020).
Ariga, K., Vinu, A., Yamauchi, Y., Ji, Q. & Hill, J. P. Nanoarchitectonics for mesoporous materials. Bull. Chem. Soc. Jpn. 85, 1–32 (2012).
Lim, H. et al. A universal approach for the synthesis of mesoporous gold, palladium and platinum films for applications in electrocatalysis. Nat. Protoc. 15, 2980–3008 (2020).
Ariga, K., Ji, Q., Nakanishi, W., Hill, J. P. & Aono, M. Nanoarchitectonics: a new materials horizon for nanotechnology. Mater. Horiz. 2, 406–413 (2015).
Gadipelli, S. & Guo, Z. X. Tuning of ZIF-derived carbon with high activity, nitrogen functionality, and yield—a case for superior CO2 capture. ChemSusChem 8, 2123–2132 (2015).
Ariga, K. & Yamauchi, Y. Nanoarchitectonics from atom to life. Chem. Asian J. 15, 718–728 (2020).
Tang, J. et al. Thermal conversion of core–shell metal–organic frameworks: a new method for selectively functionalized nanoporous hybrid carbon. J. Am. Chem. Soc. 137, 1572–1580 (2015).
Zhang, W. et al. Hollow carbon nanobubbles: monocrystalline MOF nanobubbles and their pyrolysis. Chem. Sci. 8, 3538–3546 (2017).
Kim, M. et al. KOH activated hollow ZIF-8 derived porous carbon: nanoarchitectured control for upgraded capacitive deionization and supercapacitor. ACS Appl. Mater. Interfaces 13, 52034–52043 (2021).
Wang, M. J. et al. Preparation of hollow nitrogen doped carbon via stresses induced orientation contraction. Small 14, 1804183 (2018).
Young, C. et al. Zeolitic imidazolate framework (ZIF-8) derived nanoporous carbon: the effect of carbonization temperature on the supercapacitor performance in an aqueous electrolyte. Phys. Chem. Chem. Phys. 18, 29308–29315 (2016).
Xie, Y., Kocaefe, D., Chen, C. & Kocaefe, Y. Review of research on template methods in preparation of nanomaterials. J. Nanomater. 2016, 2302595 (2016).
Malgras, V. et al. Templated synthesis for nanoarchitectured porous materials. Bull. Chem. Soc. Jpn. 88, 1171–1200 (2015).
Zhang, L., Jin, L., Liu, B. & He, J. Templated growth of crystalline mesoporous materials: from soft/hard templates to colloidal templates. Front. Chem. 7, 22 (2019).
Yang, Z., Zhang, Y. & Schnepp, Z. Soft and hard templating of graphitic carbon nitride. J. Mater. Chem. A 3, 14081–14092 (2015).
Torad, N. L. et al. Nanoarchitectured porous carbons derived from ZIFs toward highly sensitive and selective QCM sensor for hazardous aromatic vapors. J. Hazard. Mater. 405, 124248 (2021).
Cheng, N. et al. Recent development of zeolitic imidazolate frameworks (ZIFs) derived porous carbon based materials as electrocatalysts. Adv. Energy Mater. 8, 1801257 (2018).
Wang, C. et al. Gram-scale synthesis of MOF-derived superporous carbon aerogels with extraordinary adsorption capacity for organic solvents. Angew. Chem. Int. Ed. Engl. 59, 2066–2070 (2020).
Zhao, Y. et al. Nanoengineering metal–organic framework-based materials for use in electrochemical CO2 reduction reactions. Small 17, 2006590 (2021).
Salunkhe, R. R., Kaneti, Y. V., Kim, J., Kim, J. H. & Yamauchi, Y. Nanoarchitectures for metal–organic framework-derived nanoporous carbons toward supercapacitor applications. Acc. Chem. Res. 49, 2796–2806 (2016).
Xia, W. et al. Defect-rich graphene nanomesh produced by thermal exfoliation of metal–organic frameworks for the oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 58, 13354–13359 (2019).
Xu, X., Wang, H., Liu, J. & Yan, H. The applications of zeolitic imidazolate framework-8 in electrical energy storage devices: a review. J. Mater. Sci. Mater. Electron. 28, 7532–7543 (2017).
Gadipelli, S. et al. Size-related electrochemical performance in active carbon nanostructures: a MOFs-derived carbon case study. Adv. Sci. 6, 1901517 (2019).
Gadipelli, S. et al. Superior multifunctional activity of nanoporous carbons with widely tunable porosity: enhanced storage capacities for carbon-dioxide, hydrogen, water, and electric charge. Adv. Energy Mater. 10, 1903649 (2020).
Hobday, C. L. et al. Understanding the adsorption process in ZIF-8 using high pressure crystallography and computational modelling. Nat. Commun. 9, 1429 (2018).
Torad, N. L. et al. Facile synthesis of nanoporous carbons with controlled particle sizes by direct carbonization of monodispersed ZIF-8 crystals. Chem. Commun. 49, 2521–2523 (2013).
Zou, Y. et al. Tailored mesoporous inorganic biomaterials: assembly, functionalization, and drug delivery engineering. Adv. Mater. 33, 2005215 (2021).
Tang, H. et al. ZIF-8-derived hollow carbon for efficient adsorption of antibiotics. Nanomater 9, 117 (2019).
Hwang, S. et al. Hollow ZIF-8 nanoparticles improve the permeability of mixed matrix membranes for CO2/CH4 gas separation. J. Membr. Sci. 480, 11–19 (2015).
Yang, Q. et al. Metal-organic framework derived hollow N-doped porous carbon with ultrahigh concentrations of single Zn atoms for efficient carbon dioxide conversion. Angew. Chem. Int. Ed. Engl. 58, 3511–3515 (2019).
Yang, H. et al. Tunable synthesis of hollow metal-nitrogen carbon capsules for efficient oxygen reduction catalysis in proton exchange membrane fuel cells. ACS Nano 13, 8087–8098 (2019).
Jayaprakash, N. et al. Porous hollow carbon@sulfur composites for high-power lithium-sulfur batteries. Angew. Chem. Int. Ed. Engl. 50, 5904–5908 (2011).
Zhang, M. et al. Metal-organic framework derived Co3O4/C@SiO2 yolk-shell nanoreactors with enhanced catalytic performance. J. Mater. Chem. A. 6, 11226–11235 (2018).
Wang, C. et al. Metal-organic framework one-dimensional fibers as efficient catalysts for activating peroxymonosulfate. Chem. Eng. J. 330, 262–271 (2017).
Li, X. et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient fenton-like catalysis. J. Am. Chem. Soc. 140, 12469–12475 (2018).
Khan, M. A. N. et al. Metal-organic framework-derived hollow Co3O4/carbon as efficient catalyst for peroxymonosulfate activation. Chem. Eng. J. 363, 234–246 (2019).
Huang, Z. et al. Stable core-shell ZIF-8@ZIF-67 MOFs photocatalyst for highly efficient degradation of organic pollutant and hydrogen evolution. J. Mater. Res. 36, 602–614 (2021).
Chen, H. et al. Hollow-ZIF-templated formation of a ZnO@C-N-Co core-shell nanostructure for highly efficient pollutant photodegradation. J. Mater. Chem. A 5, 9937–9945 (2017).
Huang, M. et al. MOF-derived bi-metal embedded N-doped carbon polyhedral nanocages with enhanced lithium storage. J. Mater. Chem. A 5, 266–274 (2017).
Hu, Z. et al. One-step conversion from core-shell metal-organic framework materials to cobalt and nitrogen codoped carbon nanopolyhedra with hierarchically porous structure for highly efficient oxygen reduction. ACS Appl. Mater. Interfaces 9, 16109–16116 (2017).
Pan, Y. et al. Core-shell ZIF-8@ZIF-67-derived CoP nanoparticle-embedded N-doped carbon nanotube hollow polyhedron for efficient overall water splitting. J. Am. Chem. Soc. 140, 2610–2618 (2018).
Xia, W. et al. Highly ordered macroporous dual-element-doped carbon from metal-organic frameworks for catalyzing oxygen reduction. Chem. Sci. 11, 9584–9592 (2020).
Sun, T. et al. Single-atomic cobalt sites embedded in hierarchically ordered porous nitrogen-doped carbon as a superior bifunctional electrocatalyst. Proc. Natl Acad. Sci. USA 115, 12692–12697 (2018).
Kim, M. et al. Efficient lithium-ion storage using a heterostructured porous carbon framework and its in situ transmission electron microscopy study. Chem. Commun. 58, 863–866 (2022).
Liu, C. et al. Structural design and mechanism analysis of hierarchical porous carbon fibers for advanced energy and environment applications. J. Mater. Chem. A. 10, 10–49 (2022).
Lee, J. et al. Hierarchical porous carbon electrodes with sponge-like edge structures for the sensitive electrochemical detection of heavy metals. Sensors 21, 1346 (2021).
He, W. et al. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 5, 1–8 (2019).
Yan, J. et al. Multifunctional flexible membranes for sponge-like porous carbon nanofibers with high conductivity. Nat. Commun. 10, 5582 (2019).
Cravillon, J. et al. Formate modulated solvothermal synthesis of ZIF-8 investigated using time-resolved in situ X-ray diffraction and scanning electron microscopy. CrystEngComm 14, 492–498 (2012).
Avci, C. et al. Post-synthetic anisotropic wet-chemical etching of colloidal sodalite ZIF crystals. Angew. Chem. Int. Ed. Engl. 54, 14417–14421 (2015).
Salunkhe, R. R. et al. A high-performance supercapacitor cell based on ZIF-8-derived nanoporous carbon using an organic electrolyte. Chem. Commun. 52, 4764–4767 (2016).
Stoller, M. D. & Ruoff, R. S. Best practice methods for determining an electrode material’s performance of ultracapacitors. Energy Environ. Sci. 3, 1294–1301 (2010).
Marpaung, F. et al. Gram-scale synthesis of bimetallic ZIFs and their thermal conversion to nanoporous carbon materials. Nanomaterials (Basel) 9, 1796 (2019).
Gadipelli, S. et al. Switching effective oxygen reduction and evolution performance by controlled graphitization of a cobalt-nitrogen-carbon framework system. Energy Environ. Sci. 9, 1661–1667 (2016).
Acknowledgements
This research was supported by the JST-ERATO Yamauchi Materials Space-Tectonics Project (JPMJER2003). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A3A03039037). This work was also performed in part at the Queensland node of the Australian National Fabrication Facility (ANFF-Q), a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australian researchers.
Author information
Authors and Affiliations
Contributions
Y.Y. and J.N. proposed the research direction and guided the project. Y.Y. and J.N. developed the protocol. M.K., J.T. and C.Y. performed the experiments. M.K. and J.P.H. drafted the manuscript. J.K., A.K.N. and Y.S. analyzed morphologies. R.K., J.E. and A.A. did formal analysis. All authors contributed to the final writing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Srinivas Gadipelli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key papers using this protocol
Young, C. et al. Phys. Chem. Chem. Phys. 18, 29308–29315 (2016): https://doi.org/10.1039/C6CP05555A
Kim, M. et al. ACS Appl. Mater. Interfaces 13, 52034–52043 (2021): https://doi.org/10.1021/acsami.1c09107
Tang, J. et al. J. Am. Chem. Soc. 137, 1572–1580 (2015): https://doi.org/10.1021/ja511539a
Kim, M. et al. ACS Appl. Mater. Interfaces 12, 34065–34073 (2020): https://doi.org/10.1021/acsami.0c07467
Extended data
Extended Data Fig. 1 Schematic illustration of different methods and their brief procedures to prepare porous carbon precursors.
a, Template-free method. b, Hard-template method. c, Soft-template method.
Extended Data Fig. 2 Scanning electron microscopy image of ZIF-8 of different particle sizes.
a, ZIF-8 of 50 nm in diameter. b, ZIF-8 of 120 nm in diameter. c, ZIF-8 of 200 nm in diameter. Scale bars in a–c: 100 nm. d, ZIF-8 of 500 nm in diameter. e, ZIF-8 of 1.5 µm in diameter. f, ZIF-8 of 4 µm in diameter. Scale bars in d–f: 2 µm. a, d and f adapted with permission from Tang, J. et al. Thermal conversion of core–shell metal–organic frameworks: a new method for selectively functionalized nanoporous hybrid carbon. J. Am. Chem. Soc. 137, 1572–1580 (2015). Copyright 2015 American Chemical Society. b adapted with permission from Kim, M. et al. KOH activated hollow ZIF-8 derived porous carbon: nanoarchitectured control for upgraded capacitive deionization and supercapacitor. ACS Appl. Mater. Interfaces 13, 52034–52043 (2021). Copyright 2021 American Chemical Society. c adapted with permission from Kim, M. et al. Tailored nanoarchitecturing of microporous ZIF-8 to hierarchically porous double-shell carbons and their intrinsic electrochemical property. ACS Appl. Mater. Interfaces 12, 34065–34073 (2020). Copyright 2020 American Chemical Society. e adapted from ref. 45 with permission from the PCCP Owner Societies.
Extended Data Fig. 3 Physical characterization of ZIF-8 and mPDA-coated ZIF-8.
a, X-ray diffraction; b, XPS spectra; c, N2 adsorption-desorption isotherms; and d, NLDFT pore-size distributions of ZIF-8, ZIF-8@mPDA-2.5 and ZIF-8@mPDA-5.0. Adapted with permission from Kim, M. et al. Tailored nanoarchitecturing of microporous ZIF-8 to hierarchically porous double-shell carbons and their intrinsic electrochemical property. ACS Appl. Mater. Interfaces 12, 34065–34073 (2020). Copyright 2020 American Chemical Society.
Extended Data Fig. 4 Device-level demonstration of a symmetric supercapacitor by using NPC as electrode material.
a, Photograph of assembled HS test cell, schematic illustration of its cross-sectional view and SEM image of NPC on the current collector. b, Gravimetric capacitance of NPC-800, NPC-900, NPC-1,000 and activated carbon. c, Mean volumetric capacitance at 0.1 A/g (left) and mean retention of volumetric capacitance from 0.1 to 2 A/g (right) of NPC-800, NPC-900, NPC-1,000 and activated carbon, with the whiskers showing the range of data obtained from five different cells.
Supplementary information
Supplementary Information
Supplementary Method 1, Supplementary Tables 1 and 2 and Supplementary References.
Source data
Source Data Fig. 6
Pore size distribution of HPC-2.5 and HPC-5.0 (https://doi.org/10.6084/m9.figshare.18515858.v1)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, M., Xin, R., Earnshaw, J. et al. MOF-derived nanoporous carbons with diverse tunable nanoarchitectures. Nat Protoc 17, 2990–3027 (2022). https://doi.org/10.1038/s41596-022-00718-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00718-2
This article is cited by
-
Synergistic cerium oxide nanozymes: targeting DNA damage and alleviating tumor hypoxia for improved NSCLC radiotherapy efficiency
Journal of Nanobiotechnology (2024)
-
Efficient osmosis-powered production of green hydrogen
Nature Sustainability (2024)
-
Pd nanoparticles supported on porous N-doped carbon derived from ZIF-8: a facile and efficient catalyst for reduction of nitrophenols
Journal of Porous Materials (2024)
-
Solvent-pair surfactants enabled assembly of clusters and copolymers towards programmed mesoporous metal oxides
Nature Communications (2023)
-
Diffusion controlled electrochemical analysis of MoS2 and MOF derived metal oxide–carbon hybrids for high performance supercapacitors
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.