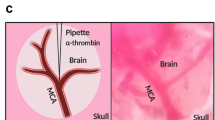
Abstract
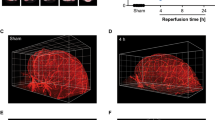
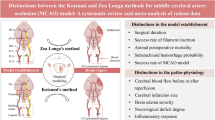
Over decades of research into the treatment of stroke, nearly all attempts to translate experimental treatments from discovery in cells and rodents to use in humans have failed. The prevailing belief is that it might be necessary to pretest pharmacological neuroprotection in higher-order brains, especially those of nonhuman primates (NHPs). Over the past few years, chemical thrombolysis and mechanical thrombectomy have been established as the standard of care for ischemic stroke in patients. The spotlight is now shifting towards emphasizing both focal ischemia and subsequent reperfusion in developing a clinically relevant stroke model in NHPs. This protocol describes an embolic model of middle cerebral artery occlusion in adult rhesus monkeys. An autologous clot is combined with a microcatheter or microwire through endovascular procedures, and reperfusion is achieved through local intra-artery thrombolysis with tissue plasminogen activator. These NHP models formed relatively stable infarct sizes, delivered predictable reperfusion and survival outcomes, and recapitulated key characteristics of patients with ischemic stroke as observed on MRI images and behavioral assays. Importantly, treated animals could survive 30 d after the surgery for post-stroke neurologic deficit analyses. Thus far, this model has been used in several translational studies. Here we describe in detail the teamwork necessary for developing stroke models of NHPs, including the preoperation preparations, endovascular surgery, postoperation management and histopathological analysis. The model can be established by the following procedures over a 45-d period, including preparation steps (14 d), endovascular operation (1 d) and evaluation steps (30 d).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets that support this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Writing Group Members et al, Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 333, 447–454 (2016).
Walker, G. B., Jadhav, A. P. & Jovin, T. G. Assessing the efficacy of endovascular therapy in stroke treatments: updates from the new generation of trials. Expert. Rev. Cardiovasc. Ther. 15, 757–766 (2017).
Goyal, M. et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomized trials. Lancet 387, 1723–1731 (2016).
Sommer, C. J. Ischemic stroke: experimental models and reality. Acta Neuropathol. 133, 245–261 (2017).
Shi, L. et al. A new era for stroke therapy: integrating neurovascular protection with optimal reperfusion. J. Cereb. Blood Flow. Metab. 38, 2073–2091 (2018).
O’Collins, V. E. et al. 1,026 experimental treatments in acute stroke. Ann. Neurol. 59, 467–477 (2006).
Fisher, M. et al. Update of the Stroke Therapy Academic Industry Roundtable preclinical recommendations. Stroke 40, 2244–2250 (2009).
Wu, D., Yue, F., Zou, C., Chan, P. & Zhang, Y. A. Analysis of glucose metabolism in cynomolgus monkeys during aging. Biogerontology 13, 147–155 (2012).
Astrup, J., Siesjö, B. K. & Symon, L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke 12, 723–725 (1981).
Cook, D. J., Teves, L. & Tymianski, M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 483, 213–217 (2012).
Marshall, J. W. et al. NXY-059, a free radical-trapping agent, substantially lessens the functional disability resulting from cerebral ischemia in a primate species. Stroke 32, 190–198 (2001).
Herrmann, A. M. et al. Large animals in neurointerventional research: a systematic review on models, techniques and their application in endovascular procedures for stroke, aneurysms and vascular malformations. J. Cereb. Blood Flow. Metab. 39, 375–394 (2019).
Hill, M. D. et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet 395, 878–887 (2020).
Mayor-Nunez, D. et al. Plasmin-resistant PSD-95 inhibitors resolve effect-modifying drug-drug interactions between alteplase and nerinetide in acute stroke. Sci. Transl. Med. 13, eabb1498 (2021).
Roitberg, B. et al. Chronic ischemic stroke model in cynomolgus monkeys: behavioral, neuroimaging and anatomical study. Neurol. Res. 25, 68–78 (2003).
Wu, D. et al. Endovascular ischemic stroke models of adult rhesus monkeys: a comparison of two endovascular methods. Sci. Rep. 6, 31608 (2016).
Wu, D. et al. Selective intraarterial brain cooling improves long-term outcomes in a non-human primate model of embolic stroke: efficacy depending on reperfusion status. J. Cereb. Blood Flow. Metab. 40, 1415–1426 (2020).
Wu, L. et al. Intranasal salvinorin A improves neurological outcome in rhesus monkey ischemic stroke model using autologous blood clot. J. Cereb. Blood Flow. Metab. 41, 723–730 (2021).
Fang, Z. et al. A MD2-perturbing peptide has therapeutic effects in rodent and rhesus monkey models of stroke. Sci. Trans. Med. 13, eabb6716 (2021).
Gao, Y. et al. Novel acute retinal artery ischemia and reperfusion model in nonhuman primates. Stroke 51, 2568–2572 (2020).
Mergenthaler, P. & Meisel, A. Do stroke models model stroke? Dis. Model. Mech. 5, 718–725 (2012).
Marshall, J. W. et al. Serial MRI, functional recovery, and long-term infarct maturation in a non-human primate model of stroke. Brain Res. Bull. 61, 577–585 (2003).
Boltze, J. et al. New mechanistic insights, novel treatment paradigms, and clinical progress in cerebrovascular diseases. Front. Aging Neurosci. 13, 623751 (2021).
Zhao, B. et al. A more consistent intraluminal rhesus monkey model of ischemic stroke. Neural Regen. Res. 9, 2087–2094 (2014).
de Crespigny, A. J. et al. Acute studies of a new primate model of reversible middle cerebral artery occlusion. J. Stroke Cerebrovasc. Dis. 14, 80–87 (2015).
Fisher, M. Endovascular therapy for basilar-artery occlusion—still waiting for answers. N. Engl. J. Med. 384, 1954–1955 (2021).
Wu, D. et al. Primate version of modified Rankin scale for classifying dysfunction in rhesus monkeys. Stroke 51, 1620–1623 (2020).
Wu, D. et al. Reperfusion plus selective intra-arterial cooling (SI-AC) improve recovery in a nonhuman primate model of stroke. Neurotherapeutics 17, 1931–1939 (2020).
Susumu, T. et al. Effects of intra-arterial urokinase on a non-human primate thromboembolic stroke model. J. Pharmacol. Sci. 100, 278–284 (2006).
Qureshi, A. I. et al. Intraarterial reteplase and intravenous abciximab for treatment of acute ischemic stroke. A preliminary feasibility and safety study in a nonhuman primate model. Neuroradiology 47, 845–854 (2005).
Yoshikawa, T. et al. Ginsenoside Rb1 reduces neurodegeneration in the peri-infarct area of a thromboembolic stroke model in non-human primates. J. Pharmacol. Sci. 107, 32–40 (2008).
Kuge, Y. et al. Serial changes in cerebral blood flow and flow-metabolism uncoupling in primates with acute thromboembolic stroke. J. Cereb. Blood Flow. Metab. 21, 202–210 (2001).
Jickling, G. C. & Sharp, F. R. Improving the translation of animal ischemic stroke studies to humans. Metab. Brain. Dis. 30, 461–467 (2005).
Yi, K. S. et al. Sustained diffusion reversal with inbore reperfusion in monkey stroke models: confirmed by prospective magnetic resonance imaging. J. Cereb. Blood Flow. Metab. 37, 2002–2012 (2017).
Li, K. et al. Pilot study of endovascular delivery of mesenchymal stromal cells in the aortic wall in a pig model. Cell Transplant. 30, 9636897211010652 (2021).
Camstra, K. M. et al. Canine model for selective and superselective cerebral intra-arterial therapy testing. Neurointervention 15, 107–116 (2020).
Kringe, L. et al. Quality and validity of large animal experiments in stroke: a systematic review. J. Cereb. Blood Flow. Metab. 40, 2152–2164 (2020).
Debatisse, J. et al. A non-human primate model of stroke reproducing endovascular thrombectomy and allowing long-term imaging and neurological read-outs. J. Cereb. Blood Flow. Metab. 41, 745–760 (2021).
Zhang, Z. et al. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation 106, 740–745 (2002).
Zhang, Z. & Chopp, M. Neural stem cells and ischemic brain. J. Stroke 18, 267–272 (2016).
Gauberti, M. et al. Thrombotic stroke in the anesthetized monkey (Macaca mulatta): characterization by MRI—a pilot study. Cerebrovasc. Dis. 33, 329–339 (2012).
Fisher, M. & Saver, J. L. Future directions of acute ischaemic stroke therapy. Lancet Neurol. 14, 758–767 (2015).
Takamatsu, H. et al. Detection of reperfusion injury using PET in a monkey model of cerebral ischemia. J. Nucl. Med. 41, 1409–1416 (2000).
Sawada, H. et al. SMTP-7, a novel small-molecule thrombolytic for ischemic stroke: a study in rodents and primates. J. Cereb. Blood Flow. Metab. 34, 235–241 (2014).
Grow, D. A., McCarrey, J. R. & Navara, C. S. Advantages of nonhuman primates as preclinical models for evaluating stem cell-based therapies for Parkinson’s disease. Stem Cell. Res. 17, 352–366 (2016).
McEntire, C. R. et al. Impaired arm function and finger dexterity in a nonhuman primate model of stroke: motor and cognitive assessments. Stroke 47, 1109–1116 (2006).
Kito, G. et al. Experimental thromboembolic stroke in cynomolgus monkeys. J. Neurosci. Methods 105, 45–53 (2001).
Cui, L. L., Golubczyk, D., Tolppanen, A. M., Boltze, J. & Jolkkonen, J. Cell therapy for ischemic stroke: are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Ann. Neurol. 86, 5–16 (2019).
Neuhaus, A. A., Couch, Y., Hadley, G. & Buchan, A. M. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain 140, 2079–2092 (2017).
Tibussek, D. et al. Severe cerebral vasospasm and childhood arterial ischemic stroke after intrathecal cytarabine. Pediatrics 137, e20152143 (2016).
Amlie-Lefond, C. & Wainwright, M. S. Childhood stroke: thinking locally, acting globally? Stroke 52, 162–163 (2021).
Chen, X. et al. An ischemic stroke model of nonhuman primates for remote lesion studies: a behavioral and neuroimaging investigation. Restor. Neurol. Neurosci. 33, 131–142 (2015).
Powers, W. J. Acute ischemic stroke. N. Engl. J. Med. 383, 252–260 (2020).
Cook, D. J. & Tymianski, M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics 9, 371–379 (2012).
Dai, P. et al. A pilot study on transient ischemic stroke induced with endothelin-1 in the rhesus monkeys. Sci. Rep. 7, 45097 (2017).
Del Zoppo, G. J. et al. Experimental acute thrombotic stroke in baboons. Stroke 17, 1254–1265 (1986).
Watanabe, O., Bremer, A. M. & West, C. R. Experimental regional cerebral ischemia in the middle cerebral artery territory in primates. Part 1: angio-anatomy and description of an experimental model with selective embolization of the internal carotid artery bifurcation. Stroke 8, 61–70 (1977).
Cook, D. J., Teves, L. & Tymianski, M. A translational paradigm for the preclinical evaluation of the stroke neuroprotectant Tat-NR2B9c in gyrencephalic nonhuman primates. Sci. Transl. Med. 4, 154ra133 (2012).
D’Arceuil, H. E., Duggan, M., He, J., Pryor, J. & de Crespigny, A. Middle cerebral artery occlusion in Macaca fascicularis: acute and chronic stroke evolution. J. Med. Primatol. 35, 78–86 (2006).
Tong, F. C. et al. An enhanced model of middle cerebral artery occlusion in nonhuman primates using an endovascular trapping technique. Am. J. Neuroradiol. 36, 2354–2359 (2015).
Zhang, X. et al. Temporal evolution of ischemic lesions in nonhuman primates: a diffusion and perfusion MRI study. PLoS ONE 10, e0117290 (2015).
Zhang, L. et al. Focal embolic cerebral ischemia in the rat. Nat. Protoc. 10, 539–547 (2015).
Yonas, H., Wolfson, S. K. Jr., Dujovny, M., Boehnke, M. & Cook, E. Selective lenticulostriate occlusion in the primate. A highly focal cerebral ischemia model. Stroke 12, 567–572 (1981).
Ciccone, A. et al. Endovascular treatment for acute ischemic stroke. N. Engl. J. Med. 368, 904–913 (2013).
Berkhemer, O. A. et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 372, 11–20 (2015).
Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 (2016).
Higashida, R. T. et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 34, 109–137 (2003).
Institute for Laboratory Animal Research. Guide for the care and use of laboratory animals. Washington, DC: National Academies Press (2011).
Won, J. et al. Assessment of hand motor function in a non-human primate model of ischemic stroke. Exp. Neurobiol. 29, 300–313 (2020).
Zhang, Z. et al. A pilot behavioural and neuroimaging investigation on photothrombotic stroke models in rhesus monkeys. J. Neurosci. Methods 362, 109291 (2021).
Sparks, D. S. et al. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat. Protoc. 15, 877–924 (2020).
Jia, J. M. et al. Control of cerebral ischemia with magnetic nanoparticles. Nat. Methods 14, 160–166 (2017).
Sneed, S. E. et al. Magnetic resonance imaging and gait analysis indicate similar outcomes between Yucatan and Landrace porcine ischemic stroke models. Front. Neurol. 11, 594954 (2021).
Cattaneo, G. F. et al. Selective intra-carotid blood cooling in acute ischemic stroke: a safety and feasibility study in an ovine stroke model. J. Cereb. Blood Flow. Metab. 41, 3097–3110 (2021).
Shazeeb, M. S. et al. Infarct evolution in a large animal model of middle cerebral artery occlusion. Transl. Stroke Res. 11, 468–480 (2020).
Kurisu, K. et al. Cofilin-actin rod formation in experimental stroke is attenuated by therapeutic hypothermia and overexpression of the inducible 70 kD inducible heat shock protein (Hsp70). Brain Circ. 5, 225–233 (2019).
Shin, H. K. et al. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain 130, 1631–1642 (2007).
Saver, J. L. et al. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke 51, 2872–2884 (2020).
Jia, L., Chopp, M., Zhang, L., Lu, M. & Zhang, Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke 41, 2071–2076 (2010).
Wu, D. et al. Selective therapeutic cooling: to maximize benefits and minimize side effects related to hypothermia. J. Cereb. Blood Flow. Metab. 42, 213–215 (2022).
McTaggart, R. A. et al. Optimization of endovascular therapy in the neuroangiography suite to achieve fast and complete (expanded treatment in cerebral ischemia 2c-3) reperfusion. Stroke 51, 1961–1968 (2020).
Bouts, M. J. et al. Magnetic resonance imaging-based cerebral tissue classification reveals distinct spatiotemporal patterns of changes after stroke in non-human primates. BMC Neurosci. 16, 91 (2015).
Van Winkle, J. A. et al. Concurrent middle cerebral artery occlusion and intra-arterial drug infusion via ipsilateral common carotid artery catheter in the rat. J. Neurosci. Methods 213, 63–69 (2013).
Tian, H. et al. Influence of occlusion site and baseline ischemic core on outcome in patients with ischemic stroke. Neurology 92, e2626–e2643 (2019).
Chamorro, Á., Lo, E. H., Renú, A., van Leyden, K. & Lyden, P. D. The future of neuroprotection in stroke. J. Neurol. Neurosurg. Psychiatry 92, 129–135 (2021).
van Leyen, K., Wang, X., Selim, M. & Lo, E. H. Opening the time window. J. Cereb. Blood Flow. Metab. 39, 2539–2540 (2019).
Liu, Y. et al. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke 38, 138–145 (2007).
Mărgăritescu, O. et al. Histopathological changes in acute ischemic stroke. Rom. J. Morphol. Embryol. 50, 327–339 (2009).
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50, e344–e418 (2019).
Li, S. et al. White matter demyelination predates axonal injury after ischemic stroke in cynomolgus monkeys. Exp. Neurol. 340, 113655 (2021).
Spetzler, R. F., Zabramski, J. M., Kaufman, B. & Yeung, H. N. Acute NMR changes during MCA occlusion: a preliminary study in primates. Stroke 14, 185–191 (1983).
Kaiser, E. E. & West, F. D. Large animal ischemic stroke models: replicating human stroke pathophysiology. Neural Regen. Res. 15, 1377–1387 (2020).
Meloni, B. P. et al. Poly-Arginine Peptide-18 (R18) reduces brain injury and improves functional outcomes in a nonhuman primate stroke model. Neurotherapeutics 17, 627–634 (2020).
Harding, J. D. Nonhuman primates and translational research: progress, opportunities, and challenges. ILAR J. 58, 141–150 (2017).
Wu, D., Chandra, A., Chen, J., Ding, Y. & Ji, X. Endovascular ischemic stroke models in nonhuman primates. Neurotherapeutics 15, 146–155 (2018).
Fukuda, S. & del Zoppo, G. J. Models of focal cerebral ischemia in the nonhuman primate. ILAR J. 44, 96–104 (2003).
Sorby-Adams, A. J., Vink, R. & Turner, R. J. Large animal models of stroke and traumatic brain injury as translational tools. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R165–R190 (2018).
Bihel, E. et al. Permanent or transient chronic ischemic stroke in the non-human primate: behavioral, neuroimaging, histological, and immunohistochemical investigations. J. Cereb. Blood Flow. Metab. 30, 273–285 (2010).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82027802, 82071466, 81871022, 82071312, 82171304 and 82071468); National Key R&D Program of China (2017YFC1308401); and the ‘mission’ talent project of Beijing Municipal Administration of Hospitals (SML20150802); Beijing Municipal Science and Technology Project (Z181100001918026). We also thank P. Coan for language editing.
Author information
Authors and Affiliations
Contributions
X.J., Y.D. and D.W. designed the experiments. D.W., J.C., L.W., C.W., X. Zhi and X. Zhang performed endovascular surgery. X.H., Z.Z., F.Y. and Shengli L. performed monkey management, including preparation and supportive treatment. X.H. and Z.Z. evaluated neurological deficit and behavior testing. J.S., Y.D. and Y.F performed HE staining and analysis. M.Z. and Siejie L. performed MRI scanning and analysis. Y.M. performed anesthetic management. D.W., H.L. and X.J. wrote and edited the manuscript together. Y.D. helped to develop the model and edited the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Youngjeon Lee, Hideo Tsukada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related Links
Key references using this protocol
Wu, D. et al. J. Cereb. Blood Flow Metab. 40, 1415–1426 (2020): https://doi.org/10.1177/0271678×20903697
Zhao, B. et al. Neural. Regen. Res. 9, 2087–2094 (2014): https://doi.org/10.4103/1673-5374.147936
Wu, D. et al. Sci. Rep. 6, 31608 (2016): https://doi.org/10.1038/srep31608
Fang, Z. et al. Sci. Transl. Med. 13, eabb6716 (2021): https://doi.org/10.1126/scitranslmed.abb6716
Wu, L. et al. J. Cereb. Blood Flow Metab. 41, 723–730 (2021): https://doi.org/10.1177/0271678×20938137
Gao, Y. et al. Stroke 51, 2568–2572 (2020): https://doi.org/10.1161/STROKEAHA.119.028809
Wu, D. et al. Stroke 51, 1620–1623 (2020): https://doi.org/10.1161/STROKEAHA.119.028108
Wu, D. et al. Neurotherapeutics 17, 1931–1939 (2020): https://doi.org/10.1007/s13311-020-00895-6
Extended data
Extended Data Fig. 1 The winged infusion set tube.
Fresh blood was collected from the model to form a whole blood clot (a red clot) in a winged infusion set tube (below).
Extended Data Fig. 2 The behavior observation cage.
The diagram of the behavior observation cage is shown below, which is larger than the living cages in the facility. It is 120 × 120 × 150 cm in size. One side is equipped with transparent tempered plexiglass for recording with a camera. One side contains screw holes for fastening behavior test equipment.
Extended Data Fig. 3 Food-pickup test equipment.
a, the schematic diagram of the equipment, including two entrances in both sides. b, Four time-recording coils (in red) are placed near entrance A and B at both sides. c, The food is place in the middle of the feeding plate. d, The total time for a complete food pickup was defined as the time period for the animal to reach out to the receptacle (record B), pick up and withdraw food (record A). The normal arm can grasp the food swiftly (<1 s), but the affected arm cannot grasp the food.
Extended Data Fig. 4 The schematic diagram of the stretcher and head holder.
a, The spineboard stretcher. b, The monkey was fixed in a spineboard stretcher in an upright position. c, The head is placed in a custom-made holder to reduce movement during endovascular surgery.
Extended Data Fig. 5 The impaired neurological functions over a 30-d observation period in a model of M1 occlusion and reperfusion.
Occlusion and reperfusion in the right MCA were achieved in this model. On the first day after stroke onset, the model was drowsy, showing no appetite for the fruit (1 in red) and no defense reaction. Grasp behavior was absent on the left side (2 in red), which was the damaged side. It did not walk or exhibit extremity movements (such as jump), and only crawled against the guardrail. On day 7, the monkey grasped the fruit (1 in red) with the nonaffected hand (right side), but the grasp was absent in the left hand (2 in red). It could crawl against the guardrail, showing minimal movement and profound weakness, without extremity movements. Facial weakness was profound with constant drooling (3 in red). On day 14, the monkey grasped the fruit (1 in red) with the nonaffected hand (right side) with some help from the affected hand (2 in red). The model exhibited a noticeable preference to turn to right side (circle in the clockwise direction, 4 in red). It could walk and sit on the rail (5 in red) and do some extremity movements (standing up and grasp the top rail, 6 in red). On day 30, the monkey grasped the fruit (1 in red) with the nonaffected hand (right side) with some help from the affected hand (2 in red), but the left arm and hand were noticeably impaired. The model could turn to left side (circle in the counterclockwise direction, 4 in red). It could do some extremity movements (standing up and grasp the top rail, 6 in red).
Extended Data Fig. 6 MR angiography examination at 24 h after stroke onset.
a, M2 permanent model. M2 branch (red) on the right side was invisible, but visible on the other side. b, M2 reperfusion model. M2 branches (white) were visible on both sides. R, right; L, left.
Supplementary information
Source data
Source Data Fig. 7
Statistical source data
Rights and permissions
About this article
Cite this article
Wu, D., Chen, J., Wu, L. et al. A clinically relevant model of focal embolic cerebral ischemia by thrombus and thrombolysis in rhesus monkeys. Nat Protoc 17, 2054–2084 (2022). https://doi.org/10.1038/s41596-022-00707-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00707-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.