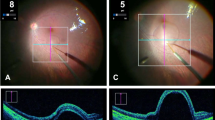
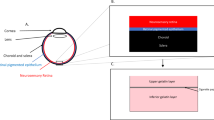
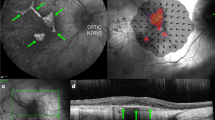
Abstract
Subretinal injection (SRI) is a widely used technique in retinal research and can be used to deliver nucleic acids, small molecules, macromolecules, viruses, cells or biomaterials such as nanobeads. Here we describe how to undertake SRI of mice. This protocol was adapted from a technique initially described for larger animals. Although SRI is a common procedure in eye research laboratories, there is no published guidance on the best practices for determining what constitutes a ‘successful’ SRI. Optimal injections are required for reproducibility of the procedure and, when carried out suboptimally, can lead to erroneous conclusions. To address this issue, we propose a standardized protocol for SRI with ‘procedure success’ defined by follow-up examination of the retina and the retinal pigmented epithelium rather than solely via intraoperative endpoints. This protocol takes 7–14 d to complete, depending on the reagent delivered. We have found, by instituting a standardized training program, that trained ophthalmologists achieve reliable proficiency in this technique after ~350 practice injections. This technique can be used to gain insights into retinal physiology and disease pathogenesis and to test the efficacy of experimental compounds in the retina or retinal pigmented epithelium.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All supporting data are available in the figures.
References
Sharma, S. et al. Pneumatic displacement of submacular hemorrhage with subretinal air and tissue plasminogen activator: initial United States experience. Ophthalmol. Retin. 2, 180–186 (2018).
Herbert, E. N., Groenewald, C. & Wong, D. Treatment of retinal folds using a modified macula relocation technique with perfluoro-hexyloctane tamponade. Br. J. Ophthalmol. 87, 921–922 (2003).
Russell, S. et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860 (2017).
Pang, J. J. et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol. Ther. 13, 565–572 (2006).
Ling, S. et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 5, 144–156 (2021).
Sun, J. et al. Protective effects of human iPS-derived retinal pigmented epithelial cells in comparison with human mesenchymal stromal cells and human neural stem cells on the degenerating retina in rd1 mice. Stem Cells 33, 1543–1553 (2015).
Kaneko, H. et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 471, 325–330 (2011).
Tarallo, V. et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 149, 847–859 (2012).
Dridi, S. et al. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc. Natl Acad. Sci. USA. 109, 13781–13786 (2012).
Kerur, N. et al. TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest. Ophthalmol. Vis. Sci. 54, 7395–7401 (2013).
Fowler, B. J. et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science 346, 1000–1003 (2014).
Kim, Y. et al. DICER1/Alu RNA dysmetabolism induces Caspase-8-mediated cell death in age-related macular degeneration. Proc. Natl Acad. Sci. USA. 111, 16082–16087 (2014).
Gelfand, B. D. et al. Iron toxicity in the retina requires Alu RNA and the NLRP3 inflammasome. Cell Rep. 11, 1686–1693 (2015).
Kerur, N. et al. cGAS drives noncanonical-inflammasome activation in age-related macular degeneration. Nat. Med. 24, 50–61 (2018).
Narendran, S. et al. Nucleoside reverse transcriptase inhibitors and Kamuvudines inhibit amyloid-beta induced retinal pigmented epithelium degeneration. Signal Transduct. Target Ther. 6, 149 (2021).
Fukuda, S. et al. Cytoplasmic synthesis of endogenous Alu complementary DNA via reverse transcription and implications in age-related macular degeneration. Proc. Natl Acad. Sci. USA 118, e2022751118 (2021).
Fukuda, S. et al. Alu complementary DNA is enriched in atrophic macular degeneration and triggers retinal pigmented epithelium toxicity via cytosolic innate immunity. Sci. Adv. 7, eabj3658 (2011).
Little, C. W. et al. Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Invest. Ophthalmol. Vis. Sci. 37, 204–211 (1996).
Peng, Y., Tang, L. & Zhou, Y. Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 58, 217–226 (2017).
Iriyama, A. et al. A2E, a component of lipofuscin, is pro-angiogenic in vivo. J. Cell Physiol. 220, 469–475 (2009).
Lyzogubov, V. V., Tytarenko, R. G., Liu, J., Bora, N. S. & Bora, P. S. Polyethylene glycol (PEG)-induced mouse model of choroidal neovascularization. J. Biol. Chem. 286, 16229–16237 (2011).
Baba, T. et al. A rat model for choroidal neovascularization using subretinal lipid hydroperoxide injection. Am. J. Pathol. 176, 3085–3097 (2010).
Narendran, S. et al. A clinical metabolite of azidothymidine inhibits experimental choroidal neovascularization and retinal pigmented epithelium degeneration. Invest. Ophthalmol. Vis. Sci. 61, 4 (2020).
Shen, D., Wen, R., Tuo, J., Bojanowski, C. M. & Chan, C. C. Exacerbation of retinal degeneration and choroidal neovascularization induced by subretinal injection of Matrigel in CCL2/MCP-1-deficient mice. Ophthalmic Res. 38, 71–73 (2006).
Jo, Y. J. et al. Establishment of a new animal model of focal subretinal fibrosis that resembles disciform lesion in advanced age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 52, 6089–6095 (2011).
Georgiadis, A. et al. Development of an optimized AAV2/5 gene therapy vector for Leber congenital amaurosis owing to defects in RPE65. Gene Ther. 23, 857–862 (2016).
Xu, H. et al. Subretinal delivery of AAV2-mediated human erythropoietin gene is protective and safe in experimental diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 55, 1519–1530 (2014).
Kim, K. et al. Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Res. 27, 419–426 (2017).
Holmgaard, A. B. et al. Targeted knockout of the Vegfa gene in the retina by subretinal injection of RNP complexes containing Cas9 protein and modified sgRNAs. Mol. Ther. 29, 191–207 (2021).
Holmgaard, A., Alsing, S., Askou, A. L. & Corydon, T. J. CRISPR gene therapy of the eye: targeted knockout of Vegfa in mouse retina by lentiviral delivery. Methods Mol. Biol. 1961, 307–328 (2019).
Wu, L. et al. Subretinal gene delivery using helper-dependent adenoviral vectors. Cell Biosci. 1, 15 (2011).
Huang, R. et al. Functional and morphological analysis of the subretinal injection of human retinal progenitor cells under Cyclosporin A treatment. Mol. Vis. 20, 1271–1280 (2014).
McGill, T. J. et al. Subretinal transplantation of human central nervous system stem cells stimulates controlled proliferation of endogenous retinal pigment epithelium. Transl. Vis. Sci. Technol. 8, 43 (2019).
Jin, Z. B. et al. Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 69, 38–56 (2019).
Maya-Vetencourt, J. F. et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol. 15, 698–708 (2020).
Fernandez-Bueno, I., Alonso-Alonso, M. L., Garcia-Gutierrez, M. T. & Diebold, Y. Reliability and reproducibility of a rodent model of choroidal neovascularization based on the subretinal injection of polyethylene glycol. Mol. Vis. 25, 194–203 (2019).
Lambert, N. G. et al. Subretinal AAV2.COMP-Ang1 suppresses choroidal neovascularization and vascular endothelial growth factor in a murine model of age-related macular degeneration. Exp. Eye Res. 145, 248–257 (2016).
Luo, L. et al. Photoreceptor avascular privilege is shielded by soluble VEGF receptor-1. eLife 2, e00324 (2013).
Luo, L. et al. Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS Nano 7, 3264–3275 (2013).
Zhang, X. et al. AAV2 delivery of Flt23k intraceptors inhibits murine choroidal neovascularization. Mol. Ther. 23, 226–234 (2015).
Cao, J. et al. A subretinal matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Invest. Ophthalmol. Vis. Sci. 51, 6009–6017 (2010).
Qiu, G. et al. A new model of experimental subretinal neovascularization in the rabbit. Exp. Eye Res. 83, 141–152 (2006).
Timmers, A. M., Zhang, H., Squitieri, A. & Gonzalez-Pola, C. Subretinal injections in rodent eyes: effects on electrophysiology and histology of rat retina. Mol. Vis. 7, 131–137 (2001).
Qi, Y. et al. Trans-corneal subretinal injection in mice and its effect on the function and morphology of the retina. PLoS One 10, e0136523 (2015).
Nickerson, J. M. et al. Subretinal delivery and electroporation in pigmented and nonpigmented adult mouse eyes. Methods Mol. Biol. 884, 53–69 (2012).
Parikh, S. et al. An alternative and validated injection method for accessing the subretinal space via a transcleral posterior approach. J. Vis. Exp. https://doi.org/10.3791/54808 (2016).
Muhlfriedel, R., Michalakis, S., Garcia Garrido, M., Biel, M. & Seeliger, M. W. Optimized technique for subretinal injections in mice. Methods Mol. Biol. 935, 343–349 (2013).
Park, S. W., Kim, J. H., Park, W. J. & Kim, J. H. Limbal approach-subretinal injection of viral vectors for gene therapy in mice retinal pigment epithelium. J. Vis. Exp. https://doi.org/10.3791/53030 (2015).
Bennett, J., Anand, V., Acland, G. M. & Maguire, A. M. Cross-species comparison of in vivo reporter gene expression after recombinant adeno-associated virus-mediated retinal transduction. Methods Enzymol. 316, 777–789 (2000).
Bartuma, H. et al. In vivo imaging of subretinal bleb-induced outer retinal degeneration in the rabbit. Invest. Ophthalmol. Vis. Sci. 56, 2423–2430 (2015).
Matsumoto, H., Miller, J. W. & Vavvas, D. G. Retinal detachment model in rodents by subretinal injection of sodium hyaluronate. J. Vis. Exp. https://doi.org/10.3791/50660 (2013).
Huang, P. et al. The learning curve of murine subretinal injection among clinically trained ophthalmic surgeons. Transl. Vis. Sci. Technol. https://doi.org/10.1167/tvst.11.3.13 (2022).
de Melo, J. & Blackshaw, S. In vivo electroporation of developing mouse retina. Methods Mol. Biol. 1715, 101–111 (2018).
Zambricki, E. A. & Dalecy, L. G. Rat sex differences in anesthesia. Comp. Med. 54, 49–53 (2004).
Pritchett-Corning, K. R., Luo, Y., Mulder, G. B. & White, W. J. Principles of rodent surgery for the new surgeon. J. Vis. Exp. https://doi.org/10.3791/2586 (2011).
Lambert, V. et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 8, 2197–2211 (2013).
Wright, C. B. et al. Chronic Dicer1 deficiency promotes atrophic and neovascular outer retinal pathologies in mice. Proc. Natl Acad. Sci. USA. 117, 2579–2587 (2020).
Wang, S. et al. DDX is an essential mediator of sterile NLRC inflammasome activation. DDX17 is an essential mediator of sterile NLRC4 inflammasome activation by retrotransposon RNAs. Sci. Immunol. (in press).
Acknowledgements
J.A. has received support from the UVA Strategic Investment Fund, NIH grants (R01EY028027, R01EY29799 and R01EY31039), DuPont Guerry, III, Professorship, and a gift from Mr. and Mrs. Eli W. Tullis; B.D.G. has received support from NIH grants (R01EY028027, R01EY031039 and R01EY032512), BrightFocus Foundation and the Owens Family Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization, P.H. and J.A.; investigation, P.H., F.P., S.N., Y.N. and S.F.; writing, P.H., F.P., S.N., K.M.M. and J.A. with assistance from I.A., P.Y., X.C., S.R.S. and B.D.G. All authors had the opportunity to discuss the results and comment on the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.A. is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has been a consultant for Allergan, Boehringer-Ingelheim, Immunovant, Olix Pharmaceuticals, Retinal Solutions and Saksin LifeSciences unrelated to this work. S.R.S. has been a consultant for 4DMT, Abbvie/Allergan, Apellis, Amgen, Centervue, Heidelberg, Iveric, Novartis, Optos, Oxurion, Regeneron and Roche/Genentech, received speaker fees from Novartis, Nidek, Carl Zeiss Meditec and Optos, and received research instruments from Carl Zeiss Meditec, Nidek, Topcon, Centervue, Optos and Heidelberg unrelated to this work; J.A. and B.D.G. are cofounders of DiceRx. J.A., S.N., I.A., F.P. and B.D.G. are named as inventors on patent applications filed by their university.
Peer review
Peer review information
Nature Protocols thanks Jin-Hyoung Kim, Fenghua Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Kaneko, H. et al. Nature 471, 325–330 (2011): https://doi.org/10.1038/nature09830
Tarallo, V. et al. Cell 149, 847–859 (2012): https://doi.org/10.1016/j.cell.2012.03.036
Kerur, N. et al. Nat. Med. 24, 50–61 (2018): https://doi.org/10.1038/nm.4450
Extended data
Extended Data Fig. 1 Custom needle parameters.
Detailed illustration of the needles designed for subretinal injection in rat (top) and mice (bottom).
Supplementary information
Supplementary Information
Supplementary Manual.
Supplementary Video 1
Subretinal injection procedure
Supplementary Video 2
Optical lens
Rights and permissions
About this article
Cite this article
Huang, P., Narendran, S., Pereira, F. et al. Subretinal injection in mice to study retinal physiology and disease. Nat Protoc 17, 1468–1485 (2022). https://doi.org/10.1038/s41596-022-00689-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00689-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.