Abstract
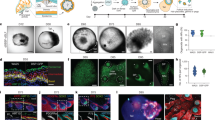
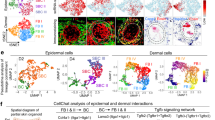
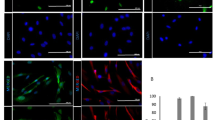
Human skin uses millions of hairs and glands distributed across the body surface to function as an external barrier, thermoregulator and stimuli sensor. The large-scale generation of human skin with these appendages would be beneficial, but is challenging. Here, we describe a detailed protocol for generating hair-bearing skin tissue entirely from a homogeneous population of human pluripotent stem cells in a three-dimensional in vitro culture system. Defined culture conditions are used over a 2-week period to induce differentiation of pluripotent stem cells to surface ectoderm and cranial neural crest cells, which give rise to the epidermis and dermis, respectively, in each organoid unit. After 60 d of incubation, the skin organoids produce hair follicles. By day ~130, the skin organoids reach full complexity and contain stratified skin layers, pigmented hair follicles, sebaceous glands, Merkel cells and sensory neurons, recapitulating the cell composition and architecture of fetal skin tissue at week 18 of gestation. Skin organoids can be maintained in culture using this protocol for up to 150 d, enabling the organoids to be used to investigate basic skin biology, model disease and, further, reconstruct or regenerate skin tissue.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Examples of results obtained are included in the figures.
Change history
23 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41596-023-00884-x
References
Dąbrowska, A. K. et al. The relationship between skin function, barrier properties, and body‐dependent factors. Ski. Res. Technol. 24, 165–174 (2018).
Kolarsick, P. A. J., Kolarsick, M. A. & Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurs. Assoc. 3, 203–213 (2011).
Nose, H., Kamijo, Y. & Masuki, S. Chapter 25—interactions between body fluid homeostasis and thermoregulation in humans. Handb. Clin. Neurol. 156, 417–429 (2018).
Woo, S.-H., Lumpkin, E. A. & Patapoutian, A. Merkel cells and neurons keep in touch. Trends Cell Biol. 25, 74–81 (2015).
Zimmerman, A., Bai, L. & Ginty, D. D. The gentle touch receptors of mammalian skin. Science 346, 950–954 (2014).
Sun, B. K., Siprashvili, Z. & Khavari, P. A. Advances in skin grafting and treatment of cutaneous wounds. Science 346, 941–945 (2014).
Karimkhani, C. et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol. 153, 406–412 (2017).
He, Z. et al. Factors affecting health-related quality of life in patients with skin disease: cross-sectional results from 8,789 patients with 16 skin diseases. Health Qual. Life Outcomes 18, 298 (2020).
Laughter, M. R. et al. The burden of skin and subcutaneous diseases in the United States from 1990 to 2017. JAMA Dermatol. 156, 874–881 (2020).
Laughter, M. R. et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017*. Brit. J. Dermatol. 184, 304–309 (2021).
Mehrmal, S., Uppal, P., Nedley, N., Giesey, R. L. & Delost, G. R. The global, regional, and national burden of psoriasis in 195 countries and territories, 1990 to 2017: a systematic analysis from the Global Burden of Disease Study 2017. J. Am. Acad. Dermatol. 84, 46–52 (2021).
Wu, X., Scott, L., Washenik, K. & Stenn, K. Full-thickness skin with mature hair follicles generated from tissue culture expanded human cells. Tissue Eng. 20, 3314–3321 (2014).
Klicznik, M. M. et al. A novel humanized mouse model to study the function of human cutaneous memory T cells in vivo in human skin. Sci. Rep. 10, 11164 (2020).
Agarwal, Y. et al. Development of humanized mouse and rat models with full-thickness human skin and autologous immune cells. Sci. Rep. 10, 14598 (2020).
Salgado, G., Ng, Y. Z., Koh, L. F., Goh, C. S. M. & Common, J. E. Human reconstructed skin xenografts on mice to model skin physiology. Differentiation 98, 14–24 (2017).
Takagi, R. et al. Bioengineering a 3D integumentary organ system from iPS cells using an in vivo transplantation model. Sci. Adv. 2, e1500887 (2016).
Zheng, Y. et al. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J. Invest. Dermatol. 124, 867–876 (2005).
Zomer, H. D. & Trentin, A. G. Skin wound healing in humans and mice: challenges in translational research. J. Dermatol. Sci. 90, 3–12 (2018).
Itoh, M. et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS ONE 8, e77673 (2013).
Yang, R. et al. Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells. Nat. Commun. 5, 3071–3071 (2014).
Gledhill, K. et al. Melanin transfer in human 3D skin equivalents generated exclusively from induced pluripotent stem cells. PLoS ONE 10, e0136713 (2015).
Abaci, H. E. et al. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat. Commun. 9, 5301 (2018).
Letsiou, S. Tracing skin aging process: a mini-review of in vitro approaches. Biogerontology 22, 261–272 (2021).
Christian, H., Hans & Yves, P. in Skin Disease Models In Vitro and Inflammatory Mechanisms: Predictability for Drug Development 187– 218 (Springer International Publishing, 2021); https://doi.org/10.1007/164_2020_428
Lee, J. et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582, 399–404 (2020).
Lee, J. & Koehler, K. R. Skin organoids: a new human model for developmental and translational research. Exp. Dermatol. 30, 613–620 (2021).
Biggs, L. C., Kim, C. S., Miroshnikova, Y. A. & Wickström, S. A. Mechanical forces in the skin: roles in tissue architecture, stability, and function. J. Invest. Dermatol. 140, 284–290 (2019).
Wong, R., Geyer, S., Weninger, W., Guimberteau, J. & Wong, J. K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 25, 92–98 (2016).
Lee, J. et al. Hair follicle development in mouse pluripotent stem cell-derived skin organoids. Cell Rep. 22, 242–254 (2018).
Koehler, K. R., Mikosz, A. M., Molosh, A. I., Patel, D. & Hashino, E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500, 217–221 (2013).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).
Koehler, K. R. et al. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat. Biotechnol. 35, 583–589 (2017).
Prummel, K. D., Nieuwenhuize, S. & Mosimann, C. The lateral plate mesoderm. Development 147, dev175059 (2020).
Guibentif, C. et al. Diverse routes toward early somites in the mouse embryo. Dev. Cell 56, 141–153.e6 (2021).
Wilson, P. A. & Hemmati-Brivanlou, A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376, 331–333 (1995).
Duverger, O. & Morasso, M. I. To grow or not to grow: hair morphogenesis and human genetic hair disorders. Semin. Cell Dev. Biol. 25, 22–33 (2014).
McCune, J. M. & Weissman, I. L. The ban on US government funding research using human fetal tissues: how does this fit with the NIH mission to advance medical science for the benefit of the citizenry? Stem Cell Rep. 13, 777–786 (2019).
Haniffa, M. et al. A roadmap for the Human Developmental Cell Atlas. Nature 597, 196–205 (2021).
Yu, Q. et al. Charting human development using a multi-endodermal organ atlas and organoid models. Cell 184, 3281–3298 (2021).
Haniffa, M. et al. Human Developmental Cell Atlas: milestones achieved and the roadmap ahead. https://doi.org/10.21203/rs.3.rs-73986/v1 (2020).
Regev, A. et al. The Human Cell Atlas. eLife 6, e27041 (2017).
Yucha, S. E. V., Tamamoto, K. A., Nguyen, H., Cairns, D. M. & Kaplan, D. L. Human skin equivalents demonstrate need for neuro-immuno-cutaneous system. Adv. Biosyst. 3, 1800283 (2019).
Meltzer, S., Santiago, C., Sharma, N. & Ginty, D. D. The cellular and molecular basis of somatosensory neuron development. Neuron 109, 3736–3757 (2021).
Andersen, J. et al. Generation of functional human 3D cortico-motor assembloids. Cell 183, 1913–1929.e26 (2020).
Wainger, B. J. et al. Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nat. Neurosci. 18, 17–24 (2015).
Oulès, B. et al. Contribution of GATA6 to homeostasis of the human upper pilosebaceous unit and acne pathogenesis. Nat. Commun. 11, 5067 (2020).
Kanwar, I. L. et al. Models for acne: a comprehensive study. Drug Discov. Ther. 12, 329–340 (2018).
Langan, S. M., Irvine, A. D. & Weidinger, S. Atopic dermatitis. Lancet 396, 345–360 (2020).
Condorelli, A. G., Dellambra, E., Logli, E., Zambruno, G. & Castiglia, D. Epidermolysis bullosa-associated squamous cell carcinoma: from pathogenesis to therapeutic perspectives. Int J. Mol. Sci. 20, 5707 (2019).
Kim, D. P., Kus, K. J. B. & Ruiz, E. Basal cell carcinoma review. Hematol. Oncol. Clin. North Am. 33, 13–24 (2019).
Davis, L. E., Shalin, S. C. & Tackett, A. J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 20, 1–14 (2019).
Que, S. K. T., Zwald, F. O. & Schmults, C. D. Cutaneous squamous cell carcinoma Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 78, 237–247 (2018).
Waldman, A. & Schmults, C. Cutaneous squamous cell carcinoma. Hematol. Oncol. Clin. North Am. 33, 1–12 (2019).
Hogue, L. & Harvey, V. M. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol. Clin. 37, 519–526 (2019).
Yum, M. K. et al. Tracing oncogene-driven remodelling of the intestinal stem cell niche. Nature 594, 442–447 (2021).
Yang, L., Mali, P., Kim-Kiselak, C. & Church, G. In Gene Correction. Methods in Molecular Biology (Methods and Protocols) (ed. Storici F.) Vol 1114, 245–267 (Humana Press, 2014); https://doi.org/10.1007/978-1-62703-761-7_16
Hendriks, D., Clevers, H. & Artegiani, B. CRISPR-Cas tools and their application in genetic engineering of human stem cells and organoids. Cell Stem Cell 27, 705–731 (2020).
Wenzel, D. et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci. Transl. Med. 6, 264ra165–264ra165 (2014).
Sebastiano, V. et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci. Transl. Med. 6, 264ra163–264ra163 (2014).
Tolar, J. et al. Induced pluripotent stem cells from individuals with recessive dystrophic epidermolysis bullosa. J. Invest. Dermatol. 131, 848–856 (2011).
Itoh, M., Kiuru, M., Cairo, M. S. & Christiano, A. M. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc. Natl Acad. Sci. USA. 108, 8797–8802 (2011).
Candi, E., Schmidt, R. & Melino, G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340 (2005).
Langbein, L., Yoshida, H., Praetzel-Wunder, S., Parry, D. A. & Schweizer, J. The keratins of the human beard hair medulla: the riddle in the middle. J. Invest. Dermatol. 130, 55–73 (2010).
Lu, C. & Fuchs, E. Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb. Perspect. Med. 4, a015222 (2014).
Lu, C. P., Polak, L., Keyes, B. E. & Fuchs, E. Spatiotemporal antagonism in mesenchymal–epithelial signaling in sweat versus hair fate decision. Science 354, aah6102 (2016).
Zhao, X. et al. Review on the vascularization of organoids and organoids-on-a-chip. Front. Bioeng. Biotechnol. 9, 637048 (2021).
Augustin, H. G., Koh, G. Y., Thurston, G. & Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 (2009).
Holloway, E. M. et al. Differentiation of human intestinal organoids with endogenous vascular endothelial cells. Dev. Cell 54, 516–528.e7 (2020).
Bar-Ephraim, Y. E., Kretzschmar, K. & Clevers, H. Organoids in immunological research. Nat. Rev. Immunol. 20, 279–293 (2020).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Tchieu, J. et al. A modular platform for differentiation of human PSCs into all major ectodermal lineages. Cell Stem Cell 21, 399–410.e7 (2017).
Volpato, V. & Webber, C. Addressing variability in iPSC-derived models of human disease: guidelines to promote reproducibility. Dis. Model Mech. 13, dmm042317 (2020).
Vigilante, A. et al. Identifying extrinsic versus intrinsic drivers of variation in cell behavior in human iPSC lines from healthy donors. Cell Rep. 26, 2078–2087.e3 (2019).
Li, W., Germain, R. N. & Gerner, M. Y. High-dimensional cell-level analysis of tissues with Ce3D multiplex volume imaging. Nat. Protoc. 14, 1708–1733 (2019).
Acknowledgements
This work was supported by the Ralph W. and Grace M. Showalter Trust (K.R.K.), the Indiana CTSI (core pilot grant UL1 TR001108 to K.R.K.), the Indiana Center for Biomedical Innovation (Technology Enhancement Grant to K.R.K.) and the NIH (grants R01AR075018 and R01DC017461 to K.R.K.). Cell lines associated with this study were stored in a facility constructed with support from the NIH (grant C06 RR020128-01). We thank M. Steinhart, C. Nist-Lund, A. Sheets, B. Cooper, S. Heller, B. Woodruff, M. Rendl, P. Rompolas, D. Spandau, J. Foley, R. Rotting, M. Haniffa, M. Boniotto, S. Sahu, S. Sharan, K. Andrykovich, R. Jaenisch, L. Biggs and S. Wickström for constructive feedback and technical assistance during the development of this method.
Author information
Authors and Affiliations
Contributions
J.L. and K.R.K. conceived the study and wrote the manuscript. W.H.v.d.V. performed whole-mount immunostaining. W.H.v.d.V., S.A.S. and C.D. performed imaging. J.L., W.H.v.d.V., S.A.S., C.D., J.K., A.P.L. and K.R.K. contributed to figure making, writing and manuscript editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.L. and K.R.K., with the Indiana University Research and Technology Corporation, have submitted a patent application covering the entire skin organoid induction method (WO2017070506A1). The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Ming Xing Lei, Yunfang Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references used in this protocol
Lee, J. et al. Nature 582, 399–404 (2020): https://doi.org/10.1038/s41586-020-2352-3
Lee, J. et al. Cell Rep. 22, 242–254 (2018): https://doi.org/10.1016/j.celrep.2017.12.007
Koehler, K. R. et al. Nat. Biotechnol. 35, 583–589 (2017): https://doi.org/10.1038/nbt.3840
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J., van der Valk, W.H., Serdy, S.A. et al. Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells. Nat Protoc 17, 1266–1305 (2022). https://doi.org/10.1038/s41596-022-00681-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00681-y
This article is cited by
-
Insights on Three Dimensional Organoid Studies for Stem Cell Therapy in Regenerative Medicine
Stem Cell Reviews and Reports (2024)
-
Dimensions of Consciousness and the Moral Status of Brain Organoids
Neuroethics (2024)
-
Bioengineered skin organoids: from development to applications
Military Medical Research (2023)
-
Mpox virus infection and drug treatment modelled in human skin organoids
Nature Microbiology (2023)
-
How developmental cell atlases inform stem cell embryo models
Nature Methods (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.