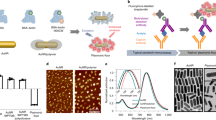
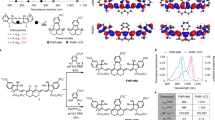
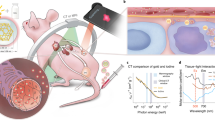
Abstract
The detection of cancer biomarkers in histological samples and blood is of paramount importance for clinical diagnosis. Current methods are limited in terms of sensitivity, hindering early detection of disease. We have overcome the shortcomings of currently available staining and fluorescence labeling methods by taking an integrative approach to establish photon-upconversion nanoparticles (UCNP) as a powerful platform for cancer detection. These nanoparticles are readily synthesized in different sizes to yield efficient and tunable short-wavelength light emission under near-infrared excitation, which eliminates optical background interference of the specimen. Here we present a protocol for the synthesis of UCNPs by high-temperature co-precipitation or seed-mediated growth by thermal decomposition, surface modification by silica or poly(ethylene glycol) that renders the particles resistant to nonspecific binding, and the conjugation of streptavidin or antibodies for biological detection. To detect blood-based biomarkers, we present an upconversion-linked immunosorbent assay for the analog and digital detection of the cancer marker prostate-specific antigen. When applied to immunocytochemistry analysis, UCNPs enable the detection of the breast cancer marker human epidermal growth factor receptor 2 with a signal-to-background ratio 50-fold higher than conventional fluorescent labels. UCNP synthesis takes 4.5 d, the preparation of the antibody–silica–UCNP conjugate takes 3 d, the streptavidin–poly(ethylene glycol)–UCNP conjugate takes 2–3 weeks, upconversion-linked immunosorbent assay takes 2–4 d and immunocytochemistry takes 8–10 h. The procedures can be performed after standard laboratory training in nanomaterials research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
This article presents representative data that support the procedures. Additional data are available in the supporting research papers (gel electrophoresis74, digital immunoassays34,41 and ICC36). Primary data underlying the figures shown in this protocol are available upon a reasonable request from the corresponding authors. Source data are provided with this paper.
References
Oliveira, H. et al. Critical considerations on the clinical translation of upconversion nanoparticles (ucnps): recommendations from the European Upconversion Network (COST Action CM1403). Adv. Healthc. Mater. 8, 1801233 (2019).
Wolfbeis, O. S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 44, 4743–4768 (2015).
Liu, T.-M., Conde, J., Lipiński, T., Bednarkiewicz, A. & Huang, C.-C. Smart NIR linear and nonlinear optical nanomaterials for cancer theranostics: prospects in photomedicine. Prog. Mater. Sci. 88, 89–135 (2017).
Zhou, B., Shi, B., Jin, D. & Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 10, 924–936 (2015).
Auzel, F. Upconversion and anti-Stokes processes with f and d ions in solids. Chem. Rev. 104, 139–174 (2004).
Liu, Q., Feng, W., Yang, T., Yi, T. & Li, F. Upconversion luminescence imaging of cells and small animals. Nat. Protoc. 8, 2033–2044 (2013).
Wang, F., Deng, R. & Liu, X. Preparation of core–shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat. Protoc. 9, 1634–1644 (2014).
Xing, Y. et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat. Protoc. 2, 1152–1165 (2007).
Cai, W. & Chen, X. Preparation of peptide-conjugated quantum dots for tumor vasculature-targeted imaging. Nat. Protoc. 3, 89–96 (2008).
Gorris, H. H., Ali, R., Saleh, S. M. & Wolfbeis, O. S. Tuning the dual emission of photon-upconverting nanoparticles for ratiometric multiplexed encoding. Adv. Mater. 23, 1652–1655 (2011).
Gorris, H. H. & Wolfbeis, O. S. Photon-upconverting nanoparticles for optical encoding and multiplexing of cells, biomolecules, and microspheres. Angew. Chem. Int. Ed. 52, 3584–3600 (2013).
Hlaváček, A., Křivánková, J., Přikryl, J. & Foret, F. Photon-upconversion barcoding with multiple barcode channels: application for droplet microfluidics. Anal. Chem. 91, 12630–12635 (2019).
Hlaváček, A., Křivánková, J., Pizúrová, N., Václavek, T. & Foret, F. Photon-upconversion barcode for monitoring an enzymatic reaction with a fluorescence reporter in droplet microfluidics. Analyst 145, 7718–7723 (2020).
Wu, S. et al. Non-blinking and photostable upconverted luminescence from single lanthanide-doped nanocrystals. Proc. Natl Acad. Sci. USA 106, 10917–10921 (2009).
Gnach, A., Lipinski, T., Bednarkiewicz, A., Rybka, J. & Capobianco, J. A. Upconverting nanoparticles: assessing the toxicity. Chem. Soc. Rev. 44, 1561–1584 (2015).
Torresan, M. F. & Wolosiuk, A. Critical aspects on the chemical stability of NaYF4-based upconverting nanoparticles for biomedical applications. ACS Appl. Bio Mater. 4, 1191–1210 (2021).
Modlitbová, P. et al. The effects of photon-upconversion nanoparticles on the growth of radish and duckweed: bioaccumulation, imaging, and spectroscopic studies. Chemosphere 225, 723–734 (2019).
Haase, M. & Schäfer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 50, 5808–5829 (2011).
Zhou, J., Liu, Q., Feng, W., Sun, Y. & Li, F. Upconversion luminescent materials: advances and applications. Chem. Rev. 115, 395–465 (2015).
Menyuk, N., Dwight, K. & Pierce, J. W. Nayf4: Yb,Er—an efficient upconversion phosphor. Appl. Phys. Lett. 21, 159–161 (1972).
Heer, S., Kömpe, K., Güdel, H.-U. & Haase, M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 16, 2102–2105 (2004).
Yi, G. et al. Synthesis, characterization, and biological application of size-controlled nanocrystalline NaYF4:Yb,Er infrared-to-visible up-conversion phosphors. Nano Lett. 4, 2191–2196 (2004).
Zeng, J.-H., Su, J., Li, Z.-H., Yan, R.-X. & Li, Y.-D. Synthesis and upconversion luminescence of hexagonal-phase NaYF4:Yb, Er3+ phosphors of controlled size and morphology. Adv. Mater. 17, 2119–2123 (2005).
Homann, C. et al. NaYF4:Yb,Er/NaYF4 core/shell nanocrystals with high upconversion luminescence quantum yield. Angew. Chem. Int. Ed. 57, 8765–8769 (2018).
Chen, B., Kong, W., Wang, N., Zhu, G. & Wang, F. Oleylamine-mediated synthesis of small naybf4 nanoparticles with tunable size. Chem. Mater. 31, 4779–4786 (2019).
Fischer, S., Swabeck, J. K. & Alivisatos, A. P. Controlled isotropic and anisotropic shell growth in β-NaLnF4 nanocrystals induced by precursor injection rate. J. Am. Chem. Soc. 139, 12325–12332 (2017).
Arppe, R. et al. Quenching of the upconversion luminescence of NaYF4:Yb3+,Er3+ and NaYF4:Yb3+,Tm3+ nanophosphors by water: the role of the sensitizer Yb3+ in non-radiative relaxation. Nanoscale 7, 11746–11757 (2015).
Würth, C., Fischer, S., Grauel, B., Alivisatos, A. P. & Resch-Genger, U. Quantum yields, surface quenching, and passivation efficiency for ultrasmall core/shell upconverting nanoparticles. J. Am. Chem. Soc. 140, 4922–4928 (2018).
Tian, B. et al. Low irradiance multiphoton imaging with alloyed lanthanide nanocrystals. Nat. Commun. 9, 3082 (2018).
Wang, J. et al. Enhancing multiphoton upconversion through energy clustering at sublattice level. Nat. Mater. 13, 157–162 (2014).
Johnson, N. J. J. et al. Direct evidence for coupled surface and concentration quenching dynamics in lanthanide-doped nanocrystals. J. Am. Chem. Soc. 139, 3275–3282 (2017).
Hlaváček, A. et al. Rapid single-step upconversion-linked immunosorbent assay for diclofenac. Microchim. Acta 184, 4159–4165 (2017).
Hlaváček, A. et al. Competitive upconversion-linked immunosorbent assay for the sensitive detection of diclofenac. Anal. Chem. 88, 6011–6017 (2016).
Farka, Z., Mickert, M. J., Hlaváček, A., Skládal, P. & Gorris, H. H. Single molecule upconversion-linked immunosorbent assay with extended dynamic range for the sensitive detection of diagnostic biomarkers. Anal. Chem. 89, 11825–11830 (2017).
Poláchová, V. et al. Click-conjugated photon-upconversion nanoparticles in an immunoassay for honeybee pathogen Melissococcus plutonius. Nanoscale 11, 8343–8351 (2019).
Farka, Z. et al. Surface design of photon-upconversion nanoparticles for high-contrast immunocytochemistry. Nanoscale 12, 8303–8313 (2020).
Peltomaa, R. et al. Competitive upconversion-linked immunoassay using peptide mimetics for the detection of the mycotoxin zearalenone. Biosens. Bioelectron. 170, (2020).
Pastucha, M. et al. Upconversion-linked immunoassay for the diagnosis of honeybee disease American foulbrood. IEEE J. Sel. Top. Quantum Electron. 27, 6900311 (2021).
Kraft, M., Würth, C., Palo, E., Soukka, T. & Resch-Genger, U. Colour-optimized quantum yields of Yb, Tm Co-doped upconversion nanocrystals. Methods Appl. Fluoresc. 7, 024001 (2019).
Zhao, J. et al. Single-nanocrystal sensitivity achieved by enhanced upconversion luminescence. Nat. Nanotechnol. 8, 729–734 (2013).
Mickert, M. J. et al. Measurement of sub-femtomolar concentrations of prostate-specific antigen through single-molecule counting with an upconversion-linked immunosorbent assay. Anal. Chem. 91, 9435–9441 (2019).
Brandmeier, J. C. et al. Effect of particle size and surface chemistry of photon-upconversion nanoparticles on analog and digital immunoassays for cardiac troponin. Adv. Healthc. Mater. 10, 2100506 (2021).
Sedlmeier, A. & Gorris, H. H. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 44, 1526–1560 (2015).
Andresen, E., Resch-Genger, U. & Schäferling, M. Surface modifications for photon-upconversion-based energy-transfer nanoprobes. Langmuir 35, 5093–5113 (2019).
Ghisaidoobe, A. B. T. & Chung, S. J. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: a focus on Förster resonance energy transfer techniques. Int. J. Mol. Sci. 15, 22518–22538 (2014).
Smith, P. K. et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85 (1985).
Rimkus, G., Bremer-Streck, S., Grüttner, C., Kaiser, W. A. & Hilger, I. Can we accurately quantify nanoparticle associated proteins when constructing high-affinity MRI molecular imaging probes? Contrast Media Mol. Imaging 6, 119–125 (2011).
Zhu, Y., Zhang, W., Li, L., Wu, C. & Xing, J. Preparation of a mixed-mode silica-based sorbent by click reaction and its application in the determination of primary aromatic amines in environmental water samples. Anal. Methods 6, 2102–2111 (2014).
Jayawardena, H. S. N., Liyanage, S. H., Rathnayake, K., Patel, U. & Yan, M. Analytical methods for characterization of nanomaterial surfaces. Anal. Chem. 93, 1889–1911 (2021).
Sapsford, K. E., Tyner, K. M., Dair, B. J., Deschamps, J. R. & Medintz, I. L. Analyzing nanomaterial bioconjugates: a review of current and emerging purification and characterization techniques. Anal. Chem. 83, 4453–4488 (2011).
Kostiv, U. et al. Versatile bioconjugation strategies of PEG-modified upconversion nanoparticles for bioanalytical applications. Biomacromolecules 21, 4502–4513 (2020).
Wilhelm, S. et al. Water dispersible upconverting nanoparticles: effects of surface modification on their luminescence and colloidal stability. Nanoscale 7, 1403–1410 (2015).
Dong, A. et al. A generalized ligand-exchange strategy enabling sequential surface functionalization of colloidal nanocrystals. J. Am. Chem. Soc. 133, 998–1006 (2011).
Bogdan, N., Vetrone, F., Ozin, G. A. & Capobianco, J. A. Synthesis of ligand-free colloidally stable water dispersible brightly luminescent lanthanide-doped upconverting nanoparticles. Nano Lett. 11, 835–840 (2011).
Sun, Y. et al. A supramolecular self-assembly strategy for upconversion nanoparticle bioconjugation. Chem. Commun. 54, 3851–3854 (2018).
Harris, J. M. & Chess, R. B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221 (2003).
Duong, H. T. T. et al. Systematic investigation of functional ligands for colloidal stable upconversion nanoparticles. RSC Adv. 8, 4842–4849 (2018).
Boyer, J.-C., Manseau, M.-P., Murray, J. I. & van Veggel, F. C. J. M. Surface modification of upconverting NaYF4 nanoparticles with PEG−phosphate ligands for NIR (800 nm) biolabeling within the biological window. Langmuir 26, 1157–1164 (2010).
Cao, P. et al. Improving lanthanide nanocrystal colloidal stability in competitive aqueous buffer solutions using multivalent PEG-phosphonate ligands. Langmuir 28, 12861–12870 (2012).
Li, L.-L., Wu, P., Hwang, K. & Lu, Y. An exceptionally simple strategy for DNA-functionalized up-conversion nanoparticles as biocompatible agents for nanoassembly, DNA delivery, and imaging. J. Am. Chem. Soc. 135, 2411–2414 (2013).
Cao, Y., Yang, Y., Shan, Y. & Huang, Z. One-pot and facile fabrication of hierarchical branched Pt–Cu nanoparticles as excellent electrocatalysts for direct methanol fuel cells. ACS Appl. Mater. Interfaces 8, 5998–6003 (2016).
Kostiv, U. et al. A simple neridronate-based surface coating strategy for upconversion nanoparticles: highly colloidally stable 125I-radiolabeled NaYF4:Yb3+/Er3+@PEG nanoparticles for multimodal in vivo tissue imaging. Nanoscale 9, 16680–16688 (2017).
Vozlič, M. et al. Formation of phosphonate coatings for improved chemical stability of upconverting nanoparticles under physiological conditions. Dalton Trans. 50, 6588–6597 (2021).
Kostiv, U. et al. Monodisperse core–shell NaYF4:Yb3+/Er3+@NaYF4:Nd3+-PEG-GGGRGDSGGGY-NH2 nanoparticlesexcitable at 808 and 980 nm: design, surface engineering, and application in life sciences. Front. Chem. 8, 497 (2020).
Kostiv, U. et al. Highly colloidally stable trimodal 125 I-radiolabeled PEG-neridronate-coated upconversion/magnetic bioimaging nanoprobes. Sci. Rep. 10, 20016 (2020).
Palo, E. et al. Effective shielding of NaYF4:Yb3+,Er3+ upconverting nanoparticles in aqueous environments using layer-by-layer assembly. Langmuir 34, 7759–7766 (2018).
Li, L.-L. et al. Biomimetic surface engineering of lanthanide-doped upconversion nanoparticles as versatile bioprobes. Angew. Chem. Int. Ed. 51, 6121–6125 (2012).
Märkl, S., Schroter, A. & Hirsch, T. Small and bright water-protected upconversion nanoparticles with long-time stability in complex, aqueous media by phospholipid membrane coating. Nano Lett. 20, 8620–8625 (2020).
Beyazit, S. et al. Versatile synthetic strategy for coating upconverting nanoparticles with polymer shells through localized photopolymerization by using the particles as internal light sources. Angew. Chem. Int. Ed. 53, 8919–8923 (2014).
Hlaváček, A., Sedlmeier, A., Skládal, P. & Gorris, H. H. Electrophoretic characterization and purification of silica-coated photon-upconverting nanoparticles and their bioconjugates. ACS Appl. Mater. Interfaces 6, 6930–6935 (2014).
Stöber, W., Fink, A. & Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 26, 62–69 (1968).
Pisani, C. et al. Experimental separation steps influence the protein content of corona around mesoporous silica nanoparticles. Nanoscale 9, 5769–5772 (2017).
Stewart, M. H. et al. Multidentate poly(ethylene glycol) ligands provide colloidal stability to semiconductor and metallic nanocrystals in extreme conditions. J. Am. Chem. Soc. 132, 9804–9813 (2010).
Hlaváček, A. et al. Large-scale purification of photon-upconversion nanoparticles by gel electrophoresis for analogue and digital bioassays. Anal. Chem. 91, 1241–1246 (2019).
Chen, G. et al. High-purity separation of gold nanoparticle dimers and trimers. J. Am. Chem. Soc. 131, 4218–4219 (2009).
Hermanson, G. Bioconjugate Techniques (Academic Press, 2008).
Anderson, N. L. & Anderson, N. G. The human plasma proteome: history, character, and diagnostic prospects. Mol. Cell. Proteom. 1, 845–867 (2002).
Cheng, H. et al. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS ONE 6, e17745 (2011).
Banys-Paluchowski, M., Krawczyk, N. & Fehm, T. Potential role of circulating tumor cell detection and monitoring in breast cancer: a review of current evidence. Front. Oncol. 6, (2016).
Rajagopal, C. & Harikumar, K. B. The origin and functions of exosomes in cancer. Front. Oncol. 8, (2018).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Hayes, J. H. & Barry, M. J. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA 311, 1143–1149 (2014).
Stephan, C., Jung, K. & Ralla, B. Current biomarkers for diagnosing of prostate cancer. Future Oncol. Lond. Engl. 11, 2743–2755 (2015).
Thaxton, C. S. et al. Nanoparticle-based bio-barcode assay redefines “undetectable” PSA and biochemical recurrence after radical prostatectomy. Proc. Natl. Acad. Sci. USA 106, 18437–18442 (2009).
Rissin, D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599 (2010).
Farka, Z. et al. Advances in optical single-molecule detection: en route to supersensitive bioaffinity assays. Angew. Chem. Int. Ed. 59, 10746–10773 (2020).
Tuffaha, M. S. A., Guski, H. & Kristiansen, G. Immunohistochemistry in Tumor Diagnostics (Springer, 2018).
Titford, M. The long history of hematoxylin. Biotech. Histochem. 80, 73–78 (2005).
Stack, E. C., Wang, C., Roman, K. A. & Hoyt, C. C. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 70, 46–58 (2014).
Coons, A. H., Creech, H. J., Jones, R. N. & Berliner, E. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J. Immunol. 45, 159–170 (1942).
Yezhelyev, M. V. et al. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 7, 657–667 (2006).
Zijlmans, H. J. M. A. A. et al. Detection of cell and tissue surface antigens using up-converting phosphors: a new reporter technology. Anal. Biochem. 267, 30–36 (1999).
Wang, M. et al. Immunolabeling and NIR-excited fluorescent imaging of HeLa cells by using NaYF4:Yb,Er upconversion nanoparticles. ACS Nano 3, 1580–1586 (2009).
Zhou, L. et al. Single-band upconversion nanoprobes for multiplexed simultaneous in situ molecular mapping of cancer biomarkers. Nat. Commun. 6, 6938 (2015).
Liu, C. et al. Detection of early primary colorectal cancer with upconversion luminescent NP-based molecular probes. Nanoscale 8, 12579–12587 (2016).
He, H. et al. Bispecific antibody-functionalized upconversion nanoprobe. Anal. Chem. 90, 3024–3029 (2018).
Dawood, S., Broglio, K., Buzdar, A. U., Hortobagyi, G. N. & Giordano, S. H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J. Clin. Oncol. 28, 92–98 (2009).
Burris, H. A. et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 29, 398–405 (2010).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 14, 461–471 (2013).
Ansari, A. A., Parchur, A. K., Thorat, N. D. & Chen, G. New advances in pre-clinical diagnostic imaging perspectives of functionalized upconversion nanoparticle-based nanomedicine. Coord. Chem. Rev. 440, 213971 (2021).
Sedlmeier, A. et al. Highly sensitive laser scanning of photon-upconverting nanoparticles on a macroscopic scale. Anal. Chem. 88, 1835–1841 (2016).
He, W. et al. Upconversion nanoparticles-based lateral flow immunoassay for point-of-care diagnosis of periodontitis. Sens. Actuators B Chem. 334, 129673 (2021).
Guo, X. et al. Single-line flow assay platform based on orthogonal emissive upconversion nanoparticles. Anal. Chem. 93, 3010–3017 (2021).
Ji, T. et al. Background-free chromatographic detection of sepsis biomarker in clinical human serum through near-infrared to near-infrared upconversion immunolabeling. ACS Nano 14, 16864–16874 (2020).
Peltomaa, R., Benito-Peña, E., Gorris, H. H. & Moreno-Bondi, M. C. Biosensing based on upconversion nanoparticles for food quality and safety applications. Analyst 146, 13–32 (2021).
Bhuckory, S. et al. Core or shell? Er3+ FRET donors in upconversion nanoparticles. Eur. J. Inorg. Chem. 2017, 5186–5195 (2017).
Siefe, C. et al. Sub-20 nm core–shell–shell nanoparticles for bright upconversion and enhanced Förster resonant energy transfer. J. Am. Chem. Soc. 141, 16997–17005 (2019).
Resch-Genger, U. & Gorris, H. H. Perspectives and challenges of photon-upconversion nanoparticles—Part I: routes to brighter particles and quantitative spectroscopic studies. Anal. Bioanal. Chem. 409, 5855–5874 (2017).
Gorris, H. H. & Resch-Genger, U. Perspectives and challenges of photon-upconversion nanoparticles—Part II: bioanalytical applications. Anal. Bioanal. Chem. 409, 5875–5890 (2017).
Chen, X., Peng, D., Ju, Q. & Wang, F. Photon upconversion in core–shell nanoparticles. Chem. Soc. Rev. 44, 1318–1330 (2015).
Del Rosal, B. & Jaque, D. Upconversion nanoparticles for in vivo applications: limitations and future perspectives. Methods Appl. Fluoresc. 7, 022001 (2019).
Näreoja, T. et al. Ratiometric sensing and imaging of intracellular pH using polyethylenimine-coated photon upconversion nanoprobes. Anal. Chem. 89, 1501–1508 (2017).
Zhan, Q. et al. Achieving high-efficiency emission depletion nanoscopy by employing cross relaxation in upconversion nanoparticles. Nat. Commun. 8, 1058 (2017).
Liu, Y. et al. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy. Nature 543, 229–233 (2017).
Lee, C. et al. Giant nonlinear optical responses from photon-avalanching nanoparticles. Nature 589, 230–235 (2021).
Li, Z. & Zhang, Y. An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF4:Yb, Er/Tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology 19, 345606 (2008).
Podhorodecki, A. et al. Percolation limited emission intensity from upconverting NaYF4:Yb3+,Er3+ nanocrystals—a single nanocrystal optical study. Nanoscale 10, 21186–21196 (2018).
Presolski, S. I., Hong, V. P. & Finn, M. G. Copper-catalyzed azide–alkyne click chemistry for bioconjugation. Curr. Protoc. Chem. Biol. 3, 153–162 (2011).
Jin, D. et al. Nanoparticles for super-resolution microscopy and single-molecule tracking. Nat. Methods 15, 415–423 (2018).
Lu, J. et al. One-step protein conjugation to upconversion nanoparticles. Anal. Chem. 87, 10406–10413 (2015).
Macpherson, S. A., Webber, G. B. & Moreno-Atanasio, R. Aggregation of nanoparticles in high ionic strength suspensions: effect of Hamaker constant and particle concentration. Adv. Powder Technol. 23, 478–484 (2012).
Lahtinen, S. et al. Disintegration of hexagonal NaYF4:Yb3+,Er3+ upconverting nanoparticles in aqueous media: the role of fluoride in solubility equilibrium. J. Phys. Chem. C. 121, 656–665 (2017).
Crowther, J. R. The ELISA Guidebook (Humana Press, 2008).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Airy, G. B. On the diffraction of an object-glass with circular aperture. Trans. Camb. Philos. Soc. 5, 283 (1835).
Acknowledgements
H.H.G. acknowledges support from the German Research Foundation (DFG: GO 1968/ 7–1 (Heisenberg Program) and GO 1968/6–1). A.H. and F.F. acknowledge institutional support grant RVO 68081715. A.H., Z.F., and P.S. acknowledge grant 21-03156S from the Czech Science Foundation. Z.F. and P.S. acknowledge the support of the Ministry of Education, Youth and Sports of the Czech Republic (MEYS CR) under the projects INTER-ACTION (LTAB19011) and CEITEC 2020 (LQ1601). D.H. acknowledges grant 21-04420S from the Czech Science Foundation. We thank P. Bouchal and P. Bouchalová for providing cell slides, N. Velychkivska for NMR measurements and V. Vykoukal for taking TEM images.
Author information
Authors and Affiliations
Contributions
A.H., Z.F., M.J.M., U.K. and J.C.B. contributed with experiments, A.H., Z.F., M.J.M., U.K., J.C.B. and H.H.G. wrote the manuscript, and P.S., F.F., D.H. and H.H.G. supervised.
Corresponding authors
Ethics declarations
Competing interests
M.J.M. was a graduate student in the Gorris group when he completed the work described in the paper and is now employed by Lumito, a company that is pursuing applications of UCNPs in IHC.
Peer review
Peer review information
Nature Protocols thanks Jiajia Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Farka, Z. et al. Nanoscale 12, 8303–8313 (2020): https://doi.org/10.1039/c9nr10568a
Mickert, M. J. et al. Anal. Chem. 91, 9435–9441 (2019): https://doi.org/10.1021/acs.analchem.9b02872
Hlaváček, A. et al. Anal. Chem. 91, 1241–1246 (2019): https://doi.org/10.1021/acs.analchem.8b04488
Supplementary information
Supplementary Information
Supplementary Table 1 and Supplementary Figs. 1–8.
Supplementary Table 2
Computation of chemicals for coating UCNPs with a carboxylated silica shell
Source data
Source Data Fig. 10
Unprocessed micrographs
Source Data Fig. 11
Unprocessed micrograph
Rights and permissions
About this article
Cite this article
Hlaváček, A., Farka, Z., Mickert, M.J. et al. Bioconjugates of photon-upconversion nanoparticles for cancer biomarker detection and imaging. Nat Protoc 17, 1028–1072 (2022). https://doi.org/10.1038/s41596-021-00670-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00670-7
This article is cited by
-
T790M mutation upconversion fluorescence biosensor via mild ATRP strategy and site-specific DNA cleavage of restriction endonuclease
Microchimica Acta (2024)
-
An updated landscape on nanotechnology-based drug delivery, immunotherapy, vaccinations, imaging, and biomarker detections for cancers: recent trends and future directions with clinical success
Discover Nano (2023)
-
Pre-activated nanoparticles with persistent luminescence for deep tumor photodynamic therapy in gallbladder cancer
Nature Communications (2023)
-
A local water molecular-heating strategy for near-infrared long-lifetime imaging-guided photothermal therapy of glioblastoma
Nature Communications (2023)
-
Photon avalanche goes multicolour
Nature Nanotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.