Abstract
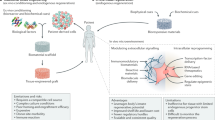
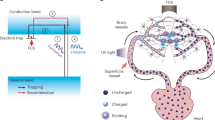
Tissue nanotransfection (TNT) is an electromotive gene transfer technology that was developed to achieve tissue reprogramming in vivo. This protocol describes how to fabricate the required hardware, commonly referred to as a TNT chip, and use it for in vivo TNT. Silicon hollow-needle arrays for TNT applications are fabricated in a standardized and reproducible way. In <1 s, these silicon hollow-needle arrays can be used to deliver plasmids to a predetermined specific depth in murine skin in response to pulsed nanoporation. Tissue nanotransfection eliminates the need to use viral vectors, minimizing the risk of genomic integration or cell transformation. The TNT chip fabrication process typically takes 5–6 d, and in vivo TNT takes 30 min. This protocol does not require specific expertise beyond a clean room equipped for basic nanofabrication processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
Data availability
The individual images used to compile the figures shown and additional examples of the types of results obtained are available from Yi Xuan upon reasonable request. The design lithography patterns we used for needle fabrication are available at https://doi.org/10.6084/m9.figshare.16528311. Source data are provided with this paper.
References
Abbasi, J. Nanochip turns skin into a bioreactor. JAMA 318, 898 (2017).
Miller, M. A. Nanotransfection brings progress that’s more than skin-deep. Sci. Transl. Med. 9, eaao4216 (2017).
Gallego-Perez, D. et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat. Nanotechnol. 12, 974–979 (2017).
Zakrewsky, M., Kumar, S. & Mitragotri, S. Nucleic acid delivery into skin for the treatment of skin disease: proofs-of-concept, potential impact, and remaining challenges. J. Control. Release 219, 445–456 (2015).
Sharei, A. et al. A vector-free microfluidic platform for intracellular delivery. Proc. Natl Acad. Sci. USA 110, 2082–2087 (2013).
Wang, Y. et al. Poking cells for efficient vector-free intracellular delivery. Nat. Commun. 5, 4466 (2014).
Pylaev, T., Vanzha, E., Avdeeva, E., Khlebtsov, B. & Khlebtsov, N. A novel cell transfection platform based on laser optoporation mediated by Au nanostar layers. J. Biophotonics 12, e201800166 (2019).
Xiong, R. H. et al. Comparison of gold nanoparticle mediated photoporation: vapor nanobubbles outperform direct heating for delivering macromolecules in live cells. ACS Nano 8, 6288–6296 (2014).
Boukany, P. E. et al. Nanochannel electroporation delivers precise amounts of biomolecules into living cells. Nat. Nanotechnol. 6, 747–754 (2011).
Shi, J. et al. A review on electroporation-based intracellular delivery. Molecules 23, 3044 (2018).
Kay, M. A., Glorioso, J. C. & Naldini, L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat. Med. 7, 33–40 (2001).
Knight, S., Collins, M. & Takeuchi, Y. Insertional mutagenesis by retroviral vectors: current concepts and methods of analysis. Curr. Gene Ther. 13, 211–227 (2013).
Sawada, S. et al. Nanogel hybrid assembly for exosome intracellular delivery: effects on endocytosis and fusion by exosome surface polymer engineering. Biomater. Sci. 8, 619–630 (2020).
Yim, N. et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 7, 12277 (2016).
Maas, S. L. N., Breakefield, X. O. & Weaver, A. M. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 27, 172–188 (2017).
Wang, Q. Y. et al. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 9, 960 (2018).
Du, J. J., Jin, J., Yan, M. & Lu, Y. F. Synthetic nanocarriers for intracellular protein delivery. Curr. Drug Metab. 13, 82–92 (2012).
Cao, Y. et al. Nontoxic nanopore electroporation for effective intracellular delivery of biological macromolecules. Proc. Natl Acad. Sci. USA 116, 7899–7904 (2019).
Gallego-Perez, D. et al. Deterministic transfection drives efficient nonviral reprogramming and uncovers reprogramming barriers. Nanomedicine 12, 399–409 (2016).
Roy, S. et al. Neurogenic tissue nanotransfection in the management of cutaneous diabetic polyneuropathy. Nanomedicine 128, 102220 (2020).
Huang, D. et al. Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array. Theranostics 8, 2361–2376 (2018).
Petchsangsai, M., Rojanarata, T., Opanasopit, P. & Ngawhirunpat, T. The combination of microneedles with electroporation and sonophoresis to enhance hydrophilic macromolecule skin penetration. Biol. Pharm. Bull. 37, 1373–1382 (2014).
Vinayakumar, K. B. et al. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J. Micromech. Microeng. 26, 065013 (2016).
McAllister, D. V. et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc. Natl Acad. Sci. USA 100, 13755–13760 (2003).
Miller, P. R. et al. Integrated carbon fiber electrodes within hollow polymer microneedles for transdermal electrochemical sensing. Biomicrofluidics 5, 13415 (2011).
Mishra, R., Maiti, T. K. & Bhattacharyya, T. K. Development of SU-8 hollow microneedles on a silicon substrate with microfluidic interconnects for transdermal drug delivery. J Micromech Microeng 28, https://doi.org/10.1088/1361-6439/aad301 (2018).
Mishra, R., Pramanick, B., Maiti, T. K. & Bhatracharyya, T. K. Glassy carbon microneedles—new transdermal drug delivery device derived from a scalable C-MEMS process. Microsyst. Nanoeng. 4, 38 (2018).
Gardeniers, H. J. G. E. et al. Silicon micromachined hollow microneedles for transdermal liquid transport. J. Microelectromech. Syst. 12, 855–862 (2003).
Li, Y. et al. Fabrication of sharp silicon hollow microneedles by deep-reactive ion etching towards minimally invasive diagnostics. Microsyst. Nanoeng. 5, 41 (2019).
Ashrf, M. et al. Design, simulation and fabrication of silicon microneedles for bio-medical applications. Trans. Electr. Eng. Electron. Commun. 9, 83–91 (2011).
Wilke, N., Mulcahy, A., Ye, S. R. & Morrissey, A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron. J. 36, 650–656 (2005).
Kang, S. K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Marty, F. et al. Advanced etching of silicon based on deep reactive ion etching for silicon high aspect ratio microstructures and three-dimensional micro- and nanostructures. Microelectron. J. 36, 673–677 (2005).
Ji, J., Tay, F. E. H., Miao, J. M. & Iliescu, C. Microfabricated silicon microneedle array for transdermal drug delivery. J. Phys. Conf. Ser. 34, 1127–1131 (2006).
Wilke, N., Hibert, C., O’Brien, J. & Morrissey, A. Silicon microneedle electrode array with temperature monitoring for electroporation. Sens. Actuat. A Phys. 123–124, 319–325 (2005).
Lai, S. L., Johnson, D. & Westerman, R. Aspect ratio dependent etching lag reduction in deep silicon etch processes. J. Vac. Sci. Technol. A 24, 1283–1288 (2006).
Tang, Y., Sandoughsaz, A., Owen, K. J. & Najafi, K. Ultra deep reactive ion etching of high aspect-ratio and thick silicon using a ramped-parameter process. J. Microelectromech. Syst. 27, 686–697 (2018).
Collins, F. Tissue nanotransfection: skin cells can be reprogrammed in vivo. https://directorsblog.nih.gov/2019/02/14/skin-cells-can-be-reprogrammed-in-vivo/ (NIH Director’s Blog, 2019).
Zhou, X. et al. Exosome-mediated crosstalk between keratinocytes and macrophages in cutaneous wound healing. ACS Nano 14, 12732–12748 (2020).
Moore, J. T. et al. Nanochannel-based poration drives benign and effective nonviral gene delivery to peripheral nerve tissue. Adv. Biosyst. 4, e2000157 (2020).
Cunningham, J. J., Ulbright, T. M., Pera, M. F. & Looijenga, L. H. Lessons from human teratomas to guide development of safe stem cell therapies. Nat. Biotechnol. 30, 849–857 (2012); erratum 31, 565 (2013).
Losordo, D. W. & Dimmeler, S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation 109, 2692–2697 (2004).
Mount, N. M., Ward, S. J., Kefalas, P. & Hyllner, J. Cell-based therapy technology classifications and translational challenges. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20150017 (2015).
Luckay, A. et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 81, 5257–5269 (2007).
Vargas, J. E. et al. Retroviral vectors and transposons for stable gene therapy: advances, current challenges and perspectives. J. Transl. Med. 14, 288 (2016).
Aihara, H. & Miyazaki, J.-i Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16, 867–870 (1998).
Mir, L. M. et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl Acad. Sci. USA 96, 4262–4267 (1999).
Lin, F. et al. Optimization of electroporation-enhanced intradermal delivery of DNA vaccine using a minimally invasive surface device. Hum. Gene Ther. Methods 23, 157–168 (2012).
Matriano, J. A. et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm. Res. 19, 63–70 (2002).
Daugimont, L. et al. Hollow microneedle arrays for intradermal drug delivery and DNA electroporation. J. Membr. Biol. 236, 117–125 (2010).
Park, J. H., Allen, M. G. & Prausnitz, M. R. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J. Control. Release 104, 51–66 (2005).
Park, J. H., Yoon, Y. K., Choi, S. O., Prausnitz, M. R. & Allen, M. G. Tapered conical polymer microneedles fabricated using an integrated lens technique for transdermal drug delivery. IEEE Trans. Biomed. Eng. 54, 903–913 (2007).
Sullivan, S. P. et al. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 16, 915–920 (2010).
van der Maaden, K. et al. Hollow microneedle-mediated micro-injections of a liposomal HPV E7(43-63) synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J. Control. Release 269, 347–354 (2018).
Kim, Y. C., Park, J. H. & Prausnitz, M. R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 64, 1547–1568 (2012).
Narayanan, S. P. & Raghavan, S. Solid silicon microneedles for drug delivery applications. Int. J. Adv. Manuf. Tech. 93, 407–422 (2017).
Xie, X. et al. Nanostraw–electroporation system for highly efficient intracellular delivery and transfection. ACS Nano 7, 4351–4358 (2013).
Cao, Y. et al. Nondestructive nanostraw intracellular sampling for longitudinal cell monitoring. Proc. Natl Acad. Sci. USA 114, E1866–E1874 (2017).
He, G. et al. Fabrication of various structures of nanostraw arrays and their applications in gene delivery. Adv. Mater. Interfaces 5, 1701535 (2018).
He, G. et al. Multifunctional branched nanostraw-electroporation platform for intracellular regulation and monitoring of circulating tumor cells. Nano Lett. 19, 7201–7209 (2019).
Tay, A. & Melosh, N. Nanostructured materials for intracellular cargo delivery. Acc. Chem. Res. 52, 2462–2471 (2019).
Wen, R. et al. Intracellular delivery and sensing system based on electroplated conductive nanostraw arrays. ACS Appl. Mater. Interfaces 11, 43936–43948 (2019).
Gill, H. S. & Prausnitz, M. R. Coated microneedles for transdermal delivery. J. Control. Release 117, 227–237 (2007).
DeMuth, P. C., Su, X., Samuel, R. E., Hammond, P. T. & Irvine, D. J. Nano-layered microneedles for transcutaneous delivery of polymer nanoparticles and plasmid DNA. Adv. Mater. 22, 4851–4856 (2010).
Kim, H. et al. Bioresorbable, miniaturized porous silicon needles on a flexible water-soluble backing for unobtrusive, sustained delivery of chemotherapy. ACS Nano 14, 7227–7236 (2020).
Chiappini, C. et al. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat. Mater. 14, 532–539 (2015).
Zhang, B., Shi, Y., Miyamoto, D., Nakazawa, K. & Miyake, T. Nanostraw membrane stamping for direct delivery of molecules into adhesive cells. Sci. Rep. 9, 6806 (2019).
Seong, H. et al. Size-tunable nanoneedle arrays for influencing stem cell morphology, gene expression, and nuclear membrane curvature. ACS Nano 14, 5371–5381 (2020).
Chen, W., Li, H., Shi, D., Liu, Z. & Yuan, W. Microneedles as a delivery system for gene therapy. Front. Pharmacol. 7, 137 (2016).
Dul, M. et al. Hydrodynamic gene delivery in human skin using a hollow microneedle device. J. Control. Release 265, 120–131 (2017).
Bolhassani, A., Khavari, A. & Orafa, Z. Electroporation—advantages and drawbacks for delivery of drug, gene and vaccine. in Application of Nanotechnology in Drug Delivery (InTech, 2014).
Huo, Z.-Y. et al. Carbon-nanotube sponges enabling highly efficient and reliable cell inactivation by low-voltage electroporation. Environ. Sci. Nano 4, 2010–2017 (2017).
Hyder, I., Eghbalsaied, S. & Kues, W. A. Systematic optimization of square-wave electroporation conditions for bovine primary fibroblasts. BMC Mol. Cell Biol. 21, 9 (2020).
Hu, Y., Werner, C. & Li, D. Electrokinetic transport through rough microchannels. Anal. Chem. 75, 5747–5758 (2003).
Fu, J. et al. Improving sidewall roughness by combined RIE-Bosch process. Mat. Sci. Semicon. Proc. 83, 186–191 (2018).
Chutani, R. K., Hasegawa, M., Maurice, V., Passilly, N. & Gorecki, C. Single-step deep reactive ion etching of ultra-deep silicon cavities with smooth sidewalls. Sens. Actuators A Phys. 208, 66 (2014).
Canatella, P. J., Karr, J. F., Petros, J. A. & Prausnitz, M. R. Quantitative study of electroporation-mediated molecular uptake and cell viability. Biophys. J. 80, 755–764 (2001).
Stewart, M. P. et al. In vitro and ex vivo strategies for intracellular delivery. Nature 538, 183–192 (2016).
Fei, Z. et al. Micronozzle array enhanced sandwich electroporation of embryonic stem cells. Anal. Chem. 82, 353–358 (2010).
Chang, L. et al. Magnetic tweezers-based 3D microchannel electroporation for high-throughput gene transfection in living cells. Small 11, 1818–1828 (2015).
Cao, Y. et al. Reply to Nathamgari et al.: nanopore electroporation for intracellular delivery of biological macromolecules. Proc. Natl Acad. Sci. USA 116, 22911 (2019).
Herrick, A., Perry, A. J. & Boswell, R. W. Etching silicon by SF6 in a continuous and pulsed power helicon reactor. J. Vac. Sci. Technol. A 21, 955–966 (2003).
Wongwanitwattana, C. et al. Precision plasma etching of Si, Ge, and Ge:P by SF6 with added O2. J. Vac. Sci. Technol. A 32, 031302 (2014).
Shikida, M., Hasada, T. & Sato, K. Fabrication of a hollow needle structure by dicing, wet etching and metal deposition. J. Micromech. Microeng. 16, 2230–2239 (2006).
Yan, G., Warner, K. S., Zhang, J., Sharma, S. & Gale, B. K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharmaceutics 391, 7–12 (2010).
Natu, R., Islam, M., Gilmore, J. & Martinez-Duarte, R. Shrinkage of SU-8 microstructures during carbonization. J. Anal. Appl. Pyrolysis 131, 17–27 (2018).
Miyazaki, J.-i. & Aihara, H. Gene transfer into muscle by electroporation in vivo. in Gene Therapy Protocols 2nd edn (ed. Morgan, J. R.). 49–62 (Springer, 2002).
Zhang, X. et al. Characteristics of liquid flow in microchannels at very low Reynolds numbers. Chem. Eng. Technol. 39, 1425–1430 (2016).
Acknowledgements
This work made use of the Pritzker Nanofabrication Facility, which receives partial support from the SHyNE Resource, a node of the National Science Foundation’s National Nanotechnology Coordinated Infrastructure (NSF ECCS-2025633). We thank Parker Evans for his help in measuring the force applied during the TNT process. This work was supported in part by NIH grant DK128845; Department of Defense grants W81XWH-21-1-0097, W81XWH-21-1-0033 and W81XWH-20-1-251 to C.K.S.; Department of Defense grant W81XWH-21-1-0047 to S.K.; and NIH grant GM143572 to Y.X.
Author information
Authors and Affiliations
Contributions
C.K.S. conceived the idea and provided design guidelines to Y.X. Y.X. finalized the designs and fabricated TNT chips with support from Z.L. and P.D. A.C., S.G., S.K. and S.R. designed and performed the TNT procedure on mice and other biological experiments. Z.L. carried out the SEM imaging and focused ion beam operation with support from D.P. M.A. integrated the TNT chip and the plasmid reservoir. All authors contributed to writing and editing the manuscript. C.K.S. supervised, resourced and led this project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Tarun Bhattacharyya, Kui Cheng, Yuanyu Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Gallego-Perez, D. et al. Nat. Nanotechnol. 12, 974–979 (2017): https://doi.org/10.1038/nnano.2017.134
Supplementary information
Supplementary Information
Supplementary Manual.
Source data
Source Data Fig. 11
Gene expression fold change raw data for Fig. 11c.
Rights and permissions
About this article
Cite this article
Xuan, Y., Ghatak, S., Clark, A. et al. Fabrication and use of silicon hollow-needle arrays to achieve tissue nanotransfection in mouse tissue in vivo. Nat Protoc 16, 5707–5738 (2021). https://doi.org/10.1038/s41596-021-00631-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00631-0
This article is cited by
-
Identification of a physiologic vasculogenic fibroblast state to achieve tissue repair
Nature Communications (2023)
-
Myogenic tissue nanotransfection improves muscle torque recovery following volumetric muscle loss
npj Regenerative Medicine (2022)
-
The start-ups taking nanoneedles into the clinic
Nature Nanotechnology (2022)
-
Modeling the gene delivery process of the needle array-based tissue nanotransfection
Nano Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.