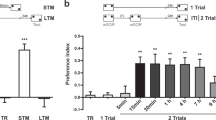
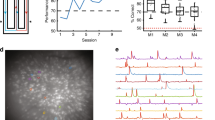
Abstract
Keeping similar memories distinct from one another is a critical cognitive process without which we would have difficulty functioning in everyday life. Memories are thought to be kept distinct through the computational mechanism of pattern separation, which reduces overlap between similar input patterns to amplify differences among stored representations. At the behavioral level, impaired pattern separation has been shown to contribute to memory deficits seen in neuropsychiatric and neurodegenerative diseases, including Alzheimer’s disease, and in normal aging. This protocol describes the use of the spontaneous location recognition (SLR) task in mice and rats to behaviorally assess spatial pattern separation ability. This two-phase spontaneous memory task assesses the extent to which animals can discriminate and remember object locations presented during the encoding phase. Using three configurations of the task, the similarity of the to-be-remembered locations can be parametrically manipulated by altering the spatial positions of objects—dissimilar, similar or extra similar—to vary the load on pattern separation. Unlike other pattern separation tasks, SLR varies the load on pattern separation during encoding, when pattern separation is thought to occur. Furthermore, SLR can be used in standard rodent behavioral facilities with basic expertise in rodent handling. The entire protocol takes ~20 d from habituation to testing of the animals on all three task configurations. By incorporating breaks between testing, and varying the objects used as landmarks, animals can be tested repeatedly, increasing experimental power by allowing for within-subjects manipulations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the main data supporting the findings of this protocol are available within the article and and/or are already published and included with permission. Extra data are available from the corresponding author upon request. Source data are provided with this paper.
References
Tulving, E. Episodic and semantic memory. in Organization of Memory (eds Tulving, E. & Donaldson, W.) 381–403 (Academic Press, 1972).
Rolls, E. The mechanisms for pattern completion and pattern separation in the hippocampus. Front. Syst. Neurosci. 7, 74 (2013).
Marr, D. & Thach, W. T. A theory of cerebellar cortex. in From the Retina to the Neocortex (ed. Vaina, L.) 11–50 (Springer, 1991).
Frankland, P. W., Cestari, V., Filipkowski, R. K., McDonald, R. J. & Silva, A. J. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav. Neurosci. 112, 863 (1998).
Gilbert, P. E., Kesner, R. P. & DeCoteau, W. E. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. J. Neurosci. 18, 804–810 (1998).
McDonald, R. J. & White, N. M. Hippocampal and nonhippocampal contributions to place learning in rats. Behav. Neurosci. 109, 579 (1995).
McTighe, S. M., Mar, A. C., Romberg, C., Bussey, T. J. & Saksida, L. M. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport 20, 881–885 (2009).
Kent, B. A., Hvoslef-Eide, M., Saksida, L. M. & Bussey, T. J. The representational–hierarchical view of pattern separation: not just hippocampus, not just space, not just memory? Neurobiol. Learn. Mem. 129, 99–106 (2016).
Bekinschtein, P. et al. Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories. Hippocampus 24, 905–911 (2014).
Clelland, C. et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 (2009).
Creer, D. J., Romberg, C., Saksida, L. M., van Praag, H. & Bussey, T. J. Running enhances spatial pattern separation in mice. Proc. Natl Acad. Sci. USA 107, 2367–2372 (2010).
Kheirbek, M. A., Klemenhagen, K. C., Sahay, A. & Hen, R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat. Neurosci. 15, 1613–1620 (2012).
Nakashiba, T. et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149, 188–201 (2012).
Sahay, A. et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 (2011).
Tronel, S. et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus 22, 292–298 (2012).
Quian Quiroga, R. No pattern separation in the human hippocampus. Trends Cogn. Sci. 24, 994–1007 (2020).
Suthana, N., Ekstrom, A. D., Yassa, M. A. & Stark, C. Pattern separation in the human hippocampus: response to Quiroga. Trends Cogn. Sci. 25, 423–424 (2021).
Berlyne, D. E. Novelty and curiosity as determinants of exploratory behaviour. Br. J. Psychol. 41, 68 (1950).
Bekinschtein, P. et al. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 5, 759–768 (2013).
Kent, B. A. et al. The orexigenic hormone acyl-ghrelin increases adult hippocampal neurogenesis and enhances pattern separation. Psychoneuroendocrinology 51, 431–439 (2015).
Miranda, M. et al. NMDA receptors and BDNF are necessary for discrimination of overlapping spatial and non-spatial memories in perirhinal cortex and hippocampus. Neurobiol. Learn. Mem. 155, 337–343 (2018).
Buyukata, C., Vukalo, M., Xu, T. J., Khore, M. A. & Reichelt, A. C. Impact of high sucrose diets on the discrimination of spatial and object memories with overlapping features. Physiol. Behav. 192, 127–133 (2018).
Gilchrist, C. P. et al. Hippocampal neurogenesis and memory in adolescence following intrauterine growth restriction. Hippocampus 31, 321–334 (2021).
Hueston, C. M. et al. Chronic interleukin-1β in the dorsal hippocampus impairs behavioural pattern separation. Brain Behav. Immun. 74, 252–264 (2018).
Reichelt, A. C., Morris, M. J. & Westbrook, R. F. Daily access to sucrose impairs aspects of spatial memory tasks reliant on pattern separation and neural proliferation in rats. Learn. Mem. 23, 386–390 (2016).
Bonafina, A. et al. GDNF and GFRα1 are required for proper integration of adult-born hippocampal neurons. Cell Rep. 29, 4308–4319. e4304 (2019).
Morales, C. et al. Dentate gyrus somatostatin cells are required for contextual discrimination during episodic memory encoding. Cereb. Cortex 31, 1046–1059 (2021).
Clark, R. E., Zola, S. M. & Squire, L. R. Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci. 20, 8853–8860 (2000).
Dix, S. L. & Aggleton, J. P. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav. Brain Res. 99, 191–200 (1999).
Gilbert, P. E., Kesner, R. P. & Lee, I. Dissociating hippocampal subregions: a double dissociation between dentate gyrus and CA1. Hippocampus 11, 626–636 (2001).
Oomen, C. A. et al. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat. Protoc. 8, 2006–2021 (2013).
Kim, C. H. et al. Trial-unique, delayed nonmatching-to-location (TUNL) touchscreen testing for mice: sensitivity to dorsal hippocampal dysfunction. Psychopharmacology 232, 3935–3945 (2015).
Talpos, J., McTighe, S., Dias, R., Saksida, L. & Bussey, T. Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol. Learn. Mem. 94, 341–352 (2010).
Hunsaker, M. R., Rosenberg, J. S. & Kesner, R. P. The role of the dentate gyrus, CA3a, b, and CA3c for detecting spatial and environmental novelty. Hippocampus 18, 1064–1073 (2008).
Van Goethem, N., Schreiber, R., Newman‐Tancredi, A., Varney, M. & Prickaerts, J. Divergent effects of the ‘biased’5‐HT 1 A receptor agonists F15599 and F13714 in a novel object pattern separation task. Br. J. Pharmacol. 172, 2532–2543 (2015).
van Goethem, N. P., van Hagen, B. T. & Prickaerts, J. Assessing spatial pattern separation in rodents using the object pattern separation task. Nat. Protoc. 13, 1763–1792 (2018).
Van Hagen, B., Van Goethem, N., Lagatta, D. & Prickaerts, J. The object pattern separation (OPS) task: a behavioral paradigm derived from the object recognition task. Behav. Brain Res. 285, 44–52 (2015).
Prusky, G. T., Harker, K. T., Douglas, R. M. & Whishaw, I. Q. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav. Brain Res. 136, 339–348 (2002).
Brown, R. E. & Wong, A. A. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn. Mem. 14, 134–144 (2007).
Wong, A. A. & Brown, R. E. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 5, 389–403 (2006).
Abbott, K. N., Morris, M. J., Westbrook, R. F. & Reichelt, A. C. Sex-specific effects of daily exposure to sucrose on spatial memory performance in male and female rats, and implications for estrous cycle stage. Physiol. Behav. 162, 52–60 (2016).
Shansky, R. M. Are hormones a “female problem” for animal research? Science 364, 825–826 (2019).
Costa, R., Tamascia, M. L., Nogueira, M. D., Casarini, D. E. & Marcondes, F. K. Handling of adolescent rats improves learning and memory and decreases anxiety. J. Am. Assoc. Lab. Anim. Sci. 51, 548–553 (2012).
Rebouças, R. C. & Schmidek, W. R. Handling and isolation in three strains of rats affect open field, exploration, hoarding and predation. Physiol. Behav. 62, 1159–1164 (1997).
Gouveia, K. & Hurst, J. L. Optimising reliability of mouse performance in behavioural testing: the major role of non-aversive handling. Sci. Rep. 7, 44999 (2017).
Deacon, R. M. Housing, husbandry and handling of rodents for behavioral experiments. Nat. Protoc. 1, 936 (2006).
Heiderstadt, K., McLaughlin, R., Wrighe, D., Walker, S. & Gomez-Sanchez, C. The effect of chronic food and water restriction on open-field behaviour and serum corticosterone levels in rats. Lab. Anim. 34, 20–28 (2000).
Brown, K. J. & Grunberg, N. E. Effects of housing on male and female rats: crowding stresses males but calms females. Physiol. Behav. 58, 1085–1089 (1995).
Kappel, S., Hawkins, P. & Mendl, M. T. To group or not to group? Good practice for housing male laboratory mice. Animals 7, 88 (2017).
Gallistel, C. R. The Organization of Learning (MIT Press, 1990).
Sousa, N., Almeida, O. & Wotjak, C. A hitchhiker’s guide to behavioral analysis in laboratory rodents. Genes Brain Behav. 5, 5–24 (2006).
Walsh, R. N. & Cummins, R. A. The open-field test: a critical review. Psychol. Bull. 83, 482 (1976).
Simon, P., Dupuis, R. & Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 61, 59–64 (1994).
Kolb, B. Some tests of response habituation in rats with discrete lesions to the orbital or medial frontal cortex. Can. J. Psychol. 28, 260 (1974).
Klein, J. et al. Lesion of the medial prefrontal cortex and the subthalamic nucleus selectively affect depression-like behavior in rats. Behav. Brain Res. 213, 73–81 (2010).
Godsil, B. P., Stefanacci, L. & Fanselow, M. S. Bright light suppresses hyperactivity induced by excitotoxic dorsal hippocampus lesions in the rat. Behav. Neurosci. 119, 1339–1352 (2005).
Broadbent, N. J., Gaskin, S., Squire, L. R. & Clark, R. E. Object recognition memory and the rodent hippocampus. Learn. Mem. 17, 5–11 (2010).
Antunes, M. & Biala, G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13, 93–110 (2012).
Pennington, Z. T. et al. ezTrack: an open-source video analysis pipeline for the investigation of animal behavior. Sci. Rep. 9, 19979 (2019).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Sridhar, V. H., Roche, D. G. & Gingins, S. Tracktor: image-based automated tracking of animal movement and behaviour. Methods Ecol. Evol. 10, 815–820 (2019).
Drai, D., Kafkafi, N., Benjamini, Y., Elmer, G. & Golani, I. Rats and mice share common ethologically relevant parameters of exploratory behavior. Behav. Brain Res. 125, 133–140 (2001).
Akkerman, S. et al. Object recognition testing: methodological considerations on exploration and discrimination measures. Behav. Brain Res. 232, 335–347 (2012).
Acknowledgements
The protocols described are those that are currently used in our laboratories, and they were written by current members of the research group. The research leading to these results has received support from: Canada First Research Excellence Fund BrainsCAN; Natural Sciences and Engineering Research Council (NSERC); Biotechnology and Biological Sciences Research Council (grant BB/G019002/1); Innovative Medicine Initiative Joint Undertaking under grant agreement number 115008, of which resources are composed of European Federation of Pharmaceutical Industries and Associations (EFPIA) in-kind contribution and financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013); Australian Research Council (DE140101301 and DP180101974).
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of the manuscript. A.C.R. coordinated this effort.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Arjan Blokland, Thomas Freret and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Bekinschtein, P. et al. Cell Rep. 5, 759–768 (2013): https://doi.org/10.1016/j.celrep.2013.09.027
Bonafina, A. et al. Cell Rep. 29, 4308–4319 (2019): https://doi.org/10.1016/j.celrep.2019.11.100
Reichelt, A.C. et al. Learn. Mem. 23, 386–390 (2016): https://doi.org/10.1101/lm.042416.116
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Source data
Source Data Fig. 5
Statistical source data and raw data.
Rights and permissions
About this article
Cite this article
Reichelt, A.C., Kramar, C.P., Ghosh-Swaby, O.R. et al. The spontaneous location recognition task for assessing spatial pattern separation and memory across a delay in rats and mice. Nat Protoc 16, 5616–5633 (2021). https://doi.org/10.1038/s41596-021-00627-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00627-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.