Abstract
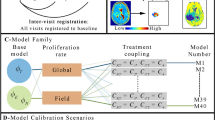
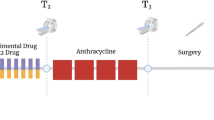
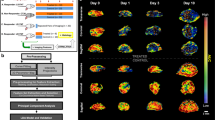
This protocol describes a complete data acquisition, analysis and computational forecasting pipeline for employing quantitative MRI data to predict the response of locally advanced breast cancer to neoadjuvant therapy in a community-based care setting. The methodology has previously been successfully applied to a heterogeneous patient population. The protocol details how to acquire the necessary images followed by registration, segmentation, quantitative perfusion and diffusion analysis, model calibration, and prediction. The data collection portion of the protocol requires ~25 min of scanning, postprocessing requires 2–3 h, and the model calibration and prediction components require ~10 h per patient depending on tumor size. The response of individual breast cancer patients to neoadjuvant therapy is forecast by application of a biophysical, reaction–diffusion mathematical model to these data. Successful application of the protocol results in coregistered MRI data from at least two scan visits that quantifies an individual tumor’s size, cellularity and vascular properties. This enables a spatially resolved prediction of how a particular patient’s tumor will respond to therapy. Expertise in image acquisition and analysis, as well as the numerical solution of partial differential equations, is required to carry out this protocol.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
We have made available one patient dataset that has been fully preprocessed (i.e., Steps 1–36) and ready for the calibration and prediction components (i.e., Steps 37–40). This will enable the interested investigator to verify that the calibration and prediction code is working on the individual investigator’s platform. The dataset is available at https://github.com/ChengyueWu/Quantitative-MRI-of-breast-cancer-patients-to-forecast-response-to-therapy.
Code availability
We have made the code for the calibration and prediction components (i.e., Steps 37–40) available without charge to anyone for academic, research, experimental or personal use. This code and license may be found at https://github.com/ChengyueWu/Quantitative-MRI-of-breast-cancer-patients-to-forecast-response-to-therapy. To distribute or make other use of the software, including commercial use, a license must be obtained from The University of Texas at Austin (by contacting licensing@otc.utexas.edu).
References
Yankeelov, T. E. et al. Toward a science of tumor forecasting for clinical oncology. Cancer Res. 75, 918–923 (2015).
Yankeelov, T. E. et al. Clinically relevant modeling of tumor growth and treatment response. Sci. Transl. Med. 5, 187ps9 (2013).
Rockne, R. C. et al. The 2019 mathematical oncology roadmap. Phys. Biol. 16, 041005 (2019).
Alfonso, J. C. L. et al. The biology and mathematical modelling of glioma invasion: a review. J. R. Soc. Interface 14, 20170490 (2017).
Chen, X., Summers, R. M. & Yao, J. Kidney tumor growth prediction by coupling reaction-diffusion and biomechanical model. IEEE Trans. Biomed. Eng. 60, 169–173 (2013).
Lorenzo, G. et al. Tissue-scale, personalized modeling and simulation of prostate cancer growth. Proc. Natl Acad. Sci. USA 113, E7663–E7671 (2016).
Lorenzo, G. et al. Computer simulations suggest that prostate enlargement due to benign prostatic hyperplasia mechanically impedes prostate cancer growth. Proc. Natl Acad. Sci. USA 116, 1152–1161 (2019).
Yuan, J. & Liu, L. Brain glioma growth model using reaction-diffusion equation with viscous stress tensor on brain MR images. Magn. Reson. Imaging 34, 114–119 (2016).
Rockne, R. et al. Predicting the efficacy of radiotherapy in individual glioblastoma patients in vivo: a mathematical modeling approach. Phys. Med. Biol. 55, 3271–3285 (2010).
Clatz, O. et al. Realistic simulation of the 3-D growth of brain tumors in MR images coupling diffusion with biomechanical deformation. IEEE Trans. Med. Imaging 24, 1334–1346 (2005).
Baldock, A. L. et al. From patient-specific mathematical neuro-oncology to precision medicine. Front. Oncol. 3, 62 (2013).
Mi, H. et al. Prediction of lung tumor evolution during radiotherapy in individual patients with PET. IEEE Trans. Med. Imaging 33, 995–1003 (2014).
Mi, H. et al. Joint tumor growth prediction and tumor segmentation on therapeutic follow-up PET images. Med. Image Anal. 23, 84–91 (2015).
Liu, Y. X. et al. Patient specific tumor growth prediction using multimodal images. Med. Image Anal. 18, 555–566 (2014).
Wong, K. C. et al. Pancreatic tumor growth prediction with elastic-growth decomposition, image-derived motion, and FDM-FEM coupling. IEEE Trans. Med. Imaging 36, 111–123 (2017).
Liu, Y. et al. Multimodal image driven patient specific tumor growth modeling. Med. Image Comput. Comput. Assist. Interv. 16, 283–290 (2013).
Wong, K. C. et al. Tumor growth prediction with reaction-diffusion and hyperelastic biomechanical model by physiological data fusion. Med. Image Anal. 25, 72–85 (2015).
Atuegwu, N. C. et al. Incorporation of diffusion-weighted magnetic resonance imaging data into a simple mathematical model of tumor growth. Phys. Med. Biol. 57, 225–240 (2012).
Atuegwu, N. C., Gore, J. C. & Yankeelov, T. E. The integration of quantitative multi-modality imaging data into mathematical models of tumors. Phys. Med. Biol. 55, 2429–2449 (2010).
Atuegwu, N. C. et al. Parameterizing the logistic model of tumor growth by DW-MRI and DCE-MRI data to predict treatment response and changes in breast cancer cellularity during neoadjuvant chemotherapy. Transl. Oncol. 6, 256–264 (2013).
Weis, J. A. et al. A mechanically coupled reaction-diffusion model for predicting the response of breast tumors to neoadjuvant chemotherapy. Phys. Med. Biol. 58, 5851–5866 (2013).
Weis, J. A. et al. Predicting the response of breast cancer to neoadjuvant therapy using a mechanically coupled reaction-diffusion model. Cancer Res. 75, 4697–4707 (2015).
Weis, J. A., Miga, M. I. & Yankeelov, T. E. Three-dimensional image-based mechanical modeling for predicting the response of breast cancer to neoadjuvant therapy. Comput. Methods Appl. Mech. Eng. 314, 494–512 (2017).
Jarrett, A. M. et al. Incorporating drug delivery into an imaging-driven, mechanics-coupled reaction diffusion model for predicting the response of breast cancer to neoadjuvant chemotherapy: theory and preliminary clinical results. Phys. Med. Biol. 63, 105015 (2018).
Jarrett, A. M. et al. Towards integration of 64Cu-DOTA-Trasztusumab PET-CT and MRI with mathematical modeling to predict response to neoadjuvant therapy in HER2+ breast cancer. Sci. Rep. 10, 20518 (2020).
Jarrett, A. M. et al. Evaluating patient-specific neoadjuvant regimens for breast cancer via a mathematical model constrained by quantitative magnetic resonance imaging data. Neoplasia 22, 820–830 (2020).
Atuegwu, N. C. et al. Integration of diffusion-weighted MRI data and a simple mathematical model to predict breast tumor cellularity during neoadjuvant chemotherapy. Magn. Reson. Med. 66, 1689–1696 (2011).
Jarrett, A. M. et al. Optimal control theory for personalized therapeutic regimens in oncology: background, history, challenges, and opportunities. J. Clin. Med. 9, 1314 (2020).
Copur, M. S. et al. Impact of the National Cancer Institute Community Cancer Centers Program on clinical trial and related activities at a community cancer center in rural Nebraska. J Oncol. Pract. 12, 67–68 (2016). e44-51.
Hormuth, D. A. et al. Mechanism-based modeling of tumor growth and treatment response constrained by multiparametric imaging data. JCO Clin. Cancer Inform. 3, 1–10 (2019).
Yankeelov, T. E., Pickens, D. R. & Price, R. R. Quantitative MRI in Cancer (CRC Press, 2012).
Huang, W. et al. Variations of dynamic contrast-enhanced magnetic resonance imaging in evaluation of breast cancer therapy response: a multicenter data analysis challenge. Transl. Oncol. 7, 153–166 (2014).
Bane, O. et al. Accuracy, repeatability, and interplatform reproducibility of T. Magn. Reson. Med. 79, 2564–2575 (2018).
Newitt, D. C. et al. Multisite concordance of apparent diffusion coefficient measurements across the NCI Quantitative Imaging Network. J. Med. Imaging (Bellingham) 5, 011003 (2018).
Bell, L. C. et al. Evaluating multisite rCBV consistency from DSC-MRI imaging protocols and postprocessing software across the NCI quantitative imaging network sites using a digital reference object (DRO). Tomography 5, 110–117 (2019).
Yankeelov, T. E. et al. Quantitative imaging in cancer clinical trials. Clin. Cancer Res. 22, 284–290 (2016).
Sorace, A. G. et al. Repeatability, reproducibility, and accuracy of quantitative MRI of the breast in the community radiology setting. J. Magn. Reson. Imaging https://doi.org/10.1002/jmri.26011(2018).
Virostko, J. et al. Magnetization transfer MRI of breast cancer in the community setting: reproducibility and preliminary results in neoadjuvant therapy. Tomography 5, 44–52 (2019).
Jones, K. M., Pagel, M. D. & Cárdenas-Rodríguez, J. Linearization improves the repeatability of quantitative dynamic contrast-enhanced MRI. Magn. Reson. Imaging 47, 16–24 (2018).
Li, X. et al. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest. Radiol. 50, 195–204 (2015).
Kim, Y. et al. Early prediction of response to neoadjuvant chemotherapy using dynamic contrast-enhanced MRI and ultrasound in breast cancer. Korean J. Radiol. 19, 682–691 (2018).
Ah-See, M. L. et al. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin. Cancer Res. 14, 6580–6589 (2008).
Padhani, A. R. et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11, 102–125 (2009).
Galbán, C. J. et al. Multi-site clinical evaluation of DW-MRI as a treatment response metric for breast cancer patients undergoing neoadjuvant chemotherapy. PLoS One 10, e0122151 (2015).
Partridge, S. C. et al. Diffusion-weighted MRI findings predict pathologic response in neoadjuvant treatment of breast cancer: the ACRIN 6698 multicenter trial. Radiology 289, 618–627 (2018).
McKnight, A. L. et al. MR elastography of breast cancer: preliminary results. AJR Am. J. Roentgenol. 178, 1411–1417 (2002).
Hormuth, D. A. et al. A mechanically coupled reaction-diffusion model that incorporates intra-tumoural heterogeneity to predict in vivo glioma growth. J. R. Soc. Interface 14, 20161010 (2017).
Hormuth, D. A. II et al. Biophysical modeling of in vivo glioma response following whole brain radiotherapy in a murine model of brain cancer. Int. J. Radiat. Oncol. Biol. Phys. 100, 1270–1279 (2018).
Hormuth, D. A. et al. Predicting in vivo glioma growth with the reaction diffusion equation constrained by quantitative magnetic resonance imaging data. Phys. Biol. 12, 046006 (2015).
Hormuth, D. A. et al. Mechanically coupled reaction-diffusion model to predict glioma growth: methodological details. Methods Mol. Biol. 1711, 225–241 (2018).
Hormuth, D. II et al. Predicting in vivo tumor growth and angiogenesis with an MRI calibrated biophysical model. Neuro-Oncol. 19, vi23 (2017).
Swanson, K. R., Alvord, E. C. & Murray, J. D. Quantifying efficacy of chemotherapy of brain tumors with homogeneous and heterogeneous drug delivery. Acta Biotheor. 50, 223–237 (2002).
Kim, M., Gillies, R. J. & Rejniak, K. A. Current advances in mathematical modeling of anti-cancer drug penetration into tumor tissues. Front. Oncol. 3, 278 (2013).
Owen, M. R. et al. Mathematical modeling predicts synergistic antitumor effects of combining a macrophage-based, hypoxia-targeted gene therapy with chemotherapy. Cancer Res. 71, 2826–2837 (2011).
Shah, A. B., Rejniak, K. A. & Gevertz, J. L. Limiting the development of anti-cancer drug resistance in a spatial model of micrometastases. Math. Biosci. Eng. 13, 1185–1206 (2016).
Bernstein, M. A., King, K. F. & Zhou, X. J. Handbook of MRI Pulse Sequences (Elsevier, 2004).
Nishimura, D. G. Principles of Magnetic Resonance Imaging (Stanford University, 1996).
Whisenant, J. G. et al. Bloch-Siegert B1-mapping improves accuracy and precision of longitudinal relaxation measurements in the breast at 3 T. Tomography 2, 250–259 (2016).
Yankeelov, T. E. & Gore, J. C. Dynamic contrast enhanced magnetic resonance imaging in oncology: theory, data acquisition, analysis, and examples. Curr. Med. Imaging Rev. 3, 91–107 (2009).
Shukla-Dave, A. et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J. Magn. Reson. Imaging 49, e101–e121 (2019).
Wu, C. et al. Quantitative analysis of vascular properties derived from ultrafast DCE-MRI to discriminate malignant and benign breast tumors. Magn. Reson. Med. 81, 2147–2160 (2019).
Chen, W., Giger, M. L. & Bick, U. A fuzzy c-means (FCM)-based approach for computerized segmentation of breast lesions in dynamic contrast-enhanced MR images. Acad. Radiol. 13, 63–72 (2006).
Li, X. et al. Validation of an algorithm for the nonrigid registration of longitudinal breast MR images using realistic phantoms. Med. Phys. 37, 2541–2552 (2010).
Gubern-Mérida, A. et al. Automated localization of breast cancer in DCE-MRI. Med. Image Anal. 20, 265–274 (2015).
Staring, M., Klein, S. & Pluim, J. P. A rigidity penalty term for nonrigid registration. Med. Phys. 34, 4098–4108 (2007).
Li, X. et al. Statistical comparison of dynamic contrast-enhanced MRI pharmacokinetic models in human breast cancer. Magn. Reson. Med. 68, 261–271 (2012).
Li, X. et al. A novel AIF tracking method and comparison of DCE-MRI parameters using individual and population-based AIFs in human breast cancer. Phys. Med. Biol. 56, 5753–5769 (2011).
Yankeelov, T. E. et al. Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magn. Reson. Imaging 23, 519–529 (2005).
Li, K. L. et al. Kinetic assessment of breast tumors using high spatial resolution signal enhancement ratio (SER) imaging. Magn. Reson. Med. 58, 572–581 (2007).
Sorace, A. G. et al. Distinguishing benign and malignant breast tumors: preliminary comparison of kinetic modeling approaches using multi-institutional dynamic contrast-enhanced MRI data from the International Breast MR Consortium 6883 trial. J. Med. Imaging (Bellingham) 5, 011019 (2018).
Li, K. L. et al. Invasive breast cancer: predicting disease recurrence by using high-spatial-resolution signal enhancement ratio imaging. Radiology 248, 79–87 (2008).
Whisenant, J. G. et al. Assessing reproducibility of diffusion-weighted magnetic resonance imaging studies in a murine model of HER2+ breast cancer. Magn. Reson. Imaging 32, 245–249 (2014).
Sugahara, T. et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging 9, 53–60 (1999).
Anderson, A. W. et al. Effects of cell volume fraction changes on apparent diffusion in human cells. Magn. Reson. Imaging 18, 689–695 (2000).
Guo, Y. et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J. Magn. Reson. Imaging 16, 172–178 (2002).
Humphries, P. D. et al. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology 245, 848–854 (2007).
Barnes, S. L. et al. Correlation of tumor characteristics derived from DCE-MRI and DW-MRI with histology in murine models of breast cancer. NMR Biomed. 28, 1345–1356 (2015).
Latour, L. L. et al. Time-dependent diffusion of water in a biological model system. Proc. Natl Acad. Sci. USA 91, 1229–1233 (1994).
van der Toorn, A. et al. Dynamic changes in water ADC, energy metabolism, extracellular space volume, and tortuosity in neonatal rat brain during global ischemia. Magn. Reson. Med. 36, 52–60 (1996).
Xu, J. et al. Magnetic resonance imaging of mean cell size in human breast tumors. Magn. Reson. Med. 83, 2002–2014 (2020).
Reynaud, O. et al. Surface-to-volume ratio mapping of tumor microstructure using oscillating gradient diffusion weighted imaging. Magn. Reson. Med. 76, 237–247 (2016).
Bedair, R. et al. Assessment of early treatment response to neoadjuvant chemotherapy in breast cancer using non-mono-exponential diffusion models: a feasibility study comparing the baseline and mid-treatment MRI examinations. Eur. Radiol. 27, 2726–2736 (2017).
Virostko, J. et al. The rate of breast fibroglandular enhancement during dynamic contrast-enhanced MRI reflects response to neoadjuvant therapy. Eur. J. Radiol. 136, 109534 (2021).
Hormuth, D. A. et al. Abstract 5487: a biologically-motivated mathematical model for forecasting chemoradiationresponse in high grade gliomas: initial results. Cancer Res. 80, 5487 (2020).
Barnes, S.L. et al. DCE- and DW-MRI as early imaging biomarkers of treatment response in a preclinical model of triple negative breast cancer. NMR Biomed. https://doi.org/10.1002/nbm.3799 (2017).
Whisenant, J. G. et al. Evaluating treatment response using DW-MRI and DCE-MRI in trastuzumab responsive and resistant HER2-overexpressing human breast cancer xenografts. Transl. Oncol. 7, 768–779 (2014).
Hormuth, D. A. et al. Calibrating a predictive model of tumor growth and angiogenesis with quantitative MRI. Ann. Biomed. Eng. 47, 1539–1551 (2019).
Hormuth, D. A., Jarrett, A. M. & Yankeelov, T. E. Forecasting tumor and vasculature response dynamics to radiation therapy via image based mathematical modeling. Radiat. Oncol. 15, 4 (2020).
Valdora, F. et al. Rapid review: radiomics and breast cancer. Breast Cancer Res. Treat. 169, 217–229 (2018).
Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: images are more than pictures, they are data. Radiology 278, 563–577 (2016).
Gillies, R. J. & Balagurunathan, Y. Perfusion MR imaging of breast cancer: insights using “habitat imaging”. Radiology 288, 36–37 (2018).
Syed, A. K. et al. Multiparametric analysis of longitudinal quantitative MRI data to identify distinct tumor habitats in preclinical models of breast cancer. Cancers (Basel) 12, 1682 (2020).
Onishi, N. et al. Ultrafast dynamic contrast-enhanced mri of the breast using compressed sensing: breast cancer diagnosis based on separate visualization of breast arteries and veins. J. Magn. Reson. Imaging 47, 97–104 (2018).
Onishi, N. et al. Ultrafast dynamic contrast-enhanced breast MRI may generate prognostic imaging markers of breast cancer. Breast Cancer Res. 22, 58 (2020).
Jarrett, A. M. et al. Mathematical models of tumor cell proliferation: a review of the literature. Expert Rev. Anticancer Ther. 18, 1271–1286 (2018).
Harris, P. A. et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009).
Harris, P. A. et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 95, 103208 (2019).
Klein, S. et al. elastix: a toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 29, 196–205 (2010).
Li, X. et al. Analyzing spatial heterogeneity in DCE- and DW-MRI parametric maps to optimize prediction of pathologic response to neoadjuvant chemotherapy in breast cancer. Transl. Oncol. 7, 14–22 (2014).
Li, X. et al. A nonrigid registration algorithm for longitudinal breast MR images and the analysis of breast tumor response. Magn. Reson. Imaging 27, 1258–1270 (2009).
Atuegwu, N. C. et al. Longitudinal, intermodality registration of quantitative breast PET and MRI data acquired before and during neoadjuvant chemotherapy: preliminary results. Med. Phys. 41, 052302 (2014).
Chung, S. et al. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn. Reson. Med. 64, 439–446 (2010).
Sung, K. et al. Simultaneous T(1) and B(1) (+) mapping using reference region variable flip angle imaging. Magn. Reson. Med. 70, 954–961 (2013).
Pineda, F. D. et al. B1 and T1 mapping of the breast with a reference tissue method. Magn. Reson. Med. 75, 1565–1573 (2016).
Yu, Z. and C. Bajaj. A fast and adaptive method for image contrast enhancement. in International Conference on Image Processing (IEEE) 2, 1001–1004 (2004).
Hagmann, P. et al. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics 26, S205–S223 (2006).
Martin, I. et al. Computer-based technique for cell aggregation analysis and cell aggregation in in vitro chondrogenesis. Cytometry 28, 141–146 (1997).
Das, C. M. et al. Dielectrophoretic segregation of different human cell types on microscope slides. Anal. Chem. 77, 2708–2719 (2005).
Ohtsu, T. et al. Clinical pharmacokinetics and pharmacodynamics of paclitaxel: a 3-hour infusion versus a 24-hour infusion. Clin. Cancer Res. 1, 599–606 (1995).
Juma, F. D., Rogers, H. J. & Trounce, J. R. Pharmacokinetics of cyclophosphamide and alkylating activity in man after intravenous and oral administration. Br. J. Clin. Pharmacol. 8, 209–217 (1979).
Green, R. F. et al. Plasma pharmacokinetics of adriamycin and adriamycinol: implications for the design of in vitro experiments and treatment protocols. Cancer Res. 43, 3417–3421 (1983).
Oguri, S. et al. Clinical pharmacokinetics of carboplatin. J. Clin. Pharmacol. 28, 208–215 (1988).
Marquardt, D. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1, 431–441 (1963).
Woodall, R. T. et al. The effects of intravoxel contrast agent diffusion on the analysis of DCE-MRI data in realistic tissue domains. Magn. Reson. Med. 80, 330–340 (2018).
Acknowledgements
We offer a sincere thank you to all the women who volunteer to participate in our studies; your strength and courage are examples for all of us. We thank N. Atuegwu, S. Eldridge, X. Li, L. Arlinghaus and J. Weis for many significant contributions to developing early versions of the techniques employed in the protocol. We thank the National Cancer Institute for support via U01 CA174706 (T.E.Y.), R01CA186193 (T.E.Y.), U24CA226110 (T.E.Y.), U01CA154602 (T.E.Y.) and R01CA240589 (A.G.S.). We thank the Cancer Prevention and Research Institute of Texas (CPRIT) for funding through RR160005. T.E.Y. is a CPRIT Scholar of Cancer Research. We thank the American Cancer Society for funding through RSG-18-006-01-CCE (A.G.S). We thank the American Association of Physicists in Medicine for funding through the 2018 Research Seed Grant (D.A.H). We thank the National Institute of Biomedical Imaging and Bioengineering for supporting A.S.K. through T32 EB007507.
Author information
Authors and Affiliations
Contributions
T.E.Y. conceived the project and obtained funding. J.C.D., J.V., A.G.S. and T.E.Y developed the procedures for the MRI acquisition with input from D.P., B.G. and S.A. A.S.K., C.W., D.A.H. and D.A.E. developed procedures for the data processing with input from A.M.J., J.V., A.G.S. and T.E.Y. A.M.J., C.W. and D.A.H. developed the procedures for generating modeling quantities with input from A.S.K. and T.E.Y. A.M.J., D.A.H. and T.E.Y. developed the procedures for tumor forecasting. A.M.J., J.C.D., J.V. and A.G.S. collected the data. A.M.J., A.S.K. and T.E.Y. organized the manuscript. All authors reviewed and edited the manuscript and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Lihua Li and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Weis, J. et al. A. Cancer Res. 74, 4697–707 (2015): https://doi.org/10.1158/0008-5472.CAN-14-2945
Jarrett, A. et al. Neoplasia. 22, 820–830 (2020): https://doi.org/10.1016/j.neo.2020.10.011
Supplementary Information
Supplementary Information
Supplementary Data 1.
Rights and permissions
About this article
Cite this article
Jarrett, A.M., Kazerouni, A.S., Wu, C. et al. Quantitative magnetic resonance imaging and tumor forecasting of breast cancer patients in the community setting. Nat Protoc 16, 5309–5338 (2021). https://doi.org/10.1038/s41596-021-00617-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00617-y
This article is cited by
-
Novel computational biology modeling system can accurately forecast response to neoadjuvant therapy in early breast cancer
Breast Cancer Research (2023)
-
A global sensitivity analysis of a mechanistic model of neoadjuvant chemotherapy for triple negative breast cancer constrained by in vitro and in vivo imaging data
Engineering with Computers (2023)
-
Quantitative multiparametric MRI predicts response to neoadjuvant therapy in the community setting
Breast Cancer Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.