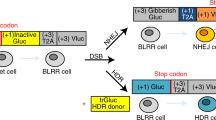
Abstract
Repair of DNA damage is a critical survival mechanism that affects susceptibility to various human diseases and represents a key target for cancer therapy. A major barrier to applying this knowledge in research and clinical translation has been the lack of efficient, quantitative functional assays for measuring DNA repair capacity in living primary cells. To overcome this barrier, we recently developed a technology termed ‘fluorescence multiplex host cell reactivation’ (FM-HCR). We describe a method for using standard molecular biology techniques to generate large quantities of FM-HCR reporter plasmids containing site-specific DNA lesions and using these reporters to assess DNA repair capacity in at least six major DNA repair pathways in live cells. We improve upon previous methodologies by (i) providing a universal workflow for generating reporter plasmids, (ii) improving yield and purity to enable large-scale studies that demand milligram quantities and (iii) reducing preparation time >ten-fold.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others

Data availability
Source data are provided with this paper. All other data supporting the approach described in this protocol are available from the corresponding authors upon reasonable request. Starting plasmids will be deposited in Adgene. Small amounts of prepared FM-HCR reporter plasmids can be shared for pilot and feasibility studies upon reasonable request.
References
Zeman, M. K. & Cimprich, K. A. Causes and consequences of replication stress. Nat. Cell Biol. 16, 2–9 (2014).
Lans, H., Hoeijmakers, J. H. J., Vermeulen, W. & Marteijn, J. A. The DNA damage response to transcription stress. Nat. Rev. Mol. Cell Biol. 20, 766–784 (2019).
Maynard, S., Fang, E. F., Scheibye-Knudsen, M., Croteau, D. L. & Bohr, V. A. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb. Perspect. Medicine 5, a025130 (2015).
Ma, J., Setton, J., Lee, N. Y., Riaz, N. & Powell, S. N. The therapeutic significance of mutational signatures from DNA repair deficiency in cancer. Nat. Commun. 9, 3292 (2018).
Kubben, N. & Misteli, T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat. Rev. Mol. Cell Biol. 18, 595–609 (2017).
Bednarski, J. J. & Sleckman, B. P. At the intersection of DNA damage and immune responses. Nat. Rev. Immunol. 19, 231–242 (2019).
Tiwari, V. & Wilson, D. M. 3rd DNA damage and associated DNA repair defects in disease and premature aging. Am. J. Hum. Genet. 105, 237–257 (2019).
Nagel, Z. D. et al. DNA repair capacity in multiple pathways predicts chemoresistance in glioblastoma multiforme. Cancer Res. 77, 198–206 (2017).
Hegi, M. E. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med 352, 997–1003 (2005).
Lord, C. J., Tutt, A. N. & Ashworth, A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu. Rev. Med. 66, 455–470 (2015).
Usanova, S. et al. Cisplatin sensitivity of testis tumour cells is due to deficiency in interstrand-crosslink repair and low ERCC1-XPF expression. Mol. Cancer 9, 248 (2010).
Link, J. T. & Overman, M. J. Immunotherapy progress in mismatch repair-deficient colorectal cancer and future therapeutic challenges. Cancer J. 22, 190–195 (2016).
Nagel, Z. D. et al. Multiplexed DNA repair assays for multiple lesions and multiple doses via transcription inhibition and transcriptional mutagenesis. Proc. Natl Acad. Sci. USA 111, E1823–E1832 (2014).
Chaim, I. A. et al. In vivo measurements of interindividual differences in DNA glycosylases and APE1 activities. Proc. Natl Acad. Sci. USA 114, E10379––E10388 (2017).
Ramos, J. M. et al. DNA repair and breast carcinoma susceptibility in women. Cancer 100, 1352–1357 (2004).
Athas, W. F., Hedayati, M. A., Matanoski, G. M., Farmer, E. R. & Grossman, L. Development and field-test validation of an assay for DNA-repair in circulating human lymphocytes. Cancer Res. 51, 5786–5793 (1991).
Qiao, Y. W. et al. Rapid assessment of repair of ultraviolet DNA damage with a modified host-cell reactivation assay using a luciferase reporter gene and correlation with polymorphisms of DNA repair genes in normal human lymphocytes. Mutat. Res. 509, 165–174 (2002).
Brégeon, D. & Doetsch, P. W. Transcriptional mutagenesis: causes and involvement in tumour development. Nat. Rev. Cancer 11, 218–227 (2011).
You, H. J., Viswanathan, A. & Doetsch, P. W. In vivo technique for determining transcriptional mutagenesis. Methods 22, 120–126 (2000).
Burger, K. et al. The influence of folic acid depletion on the Nucleotide Excision Repair capacity of human dermal fibroblasts measured by a modified Host Cell Reactivation Assay. Biofactors 31, 181–190 (2007).
Raetz, A. G. et al. Cancer-associated variants and a common polymorphism of MUTYH exhibit reduced repair of oxidative DNA damage using a GFP-based assay in mammalian cells. Carcinogenesis 33, 2301–2309 (2012).
Gunn, A. & Stark, J. M. I-SceI-based assays to examine distinct repair outcomes of mammalian chromosomal double strand breaks. Methods Mol. Biol. 920, 379–391 (2012).
Mao, Z., Bozzella, M., Seluanov, A. & Gorbunova, V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair (Amst.) 7, 1765–1771 (2008).
Pierce, A. J., Johnson, R. D., Thompson, L. H. & Jasin, M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13, 2633–2638 (1999).
Stark, J. M., Pierce, A. J., Oh, J., Pastink, A. & Jasin, M. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol. Cell Biol. 24, 9305–9316 (2004).
Burns, J. A., Dreij, K., Cartularo, L. & Scicchitano, D. A. O6-Methylguanine induces altered proteins at the level of transcription in human cells. Nucleic Acids Res. 38, 8178–8187 (2010).
Nagel, Z. D. et al. Fluorescent reporter assays provide direct, accurate, quantitative measurements of MGMT status in human cells. PLoS ONE 14, e0208341 (2019).
Lei, X. F., Zhu, Y., Tomkinson, A. & Sun, L. Z. Measurement of DNA mismatch repair activity in live cells. Nucleic Acids Res. 32, e100 (2004).
Johnson, J. M. & Latimer, J. J. Analysis of DNA repair using transfection-based host cell reactivation. in Molecular Toxicology Protocols (eds Keohavong, P. & Grant, G. G.) 321–335 (Humana Press, 2005).
Baerenfaller, K., Fischer, F. & Jiricny, J. Characterization of the ‘mismatch repairosome’ and its role in the processing of modified nucleosides in vitro. in DNA Repair, Part A (eds Campbell, J. & Modrich, P.) 285–303 (Academic Press, 2006).
Bregeon, D. & Doetsch, P. W. Reliable method for generating double-stranded DNA vectors containing site-specific base modifications. Biotechniques 37, 760–762 (2004). 764, 766.
Green, C. L., Loechler, E. L., Fowler, K. W. & Essigmann, J. M. Construction and characterization of extrachromosomal probes for mutagenesis by carcinogens: site-specific incorporation of O6-methylguanine into viral and plasmid genomes. Proc. Natl Acad. Sci. USA 81, 13–17 (1984).
Moore, S. et al. The CHD6 chromatin remodeler is an oxidative DNA damage response factor. Nat. Commun. 10, 241 (2019).
Lee, K. J. et al. Defective base excision repair in the response to DNA damaging agents in triple negative breast cancer. PLoS ONE 14, e0223725 (2019).
Xiao, A. Y. et al. Sodium sulfide selectively induces oxidative stress, DNA damage, and mitochondrial dysfunction and radiosensitizes glioblastoma (GBM) cells. Redox Biol. 26, 101220 (2019).
Chan, E. M. et al. WRN helicase is a synthetic lethal target in microsatellite unstable cancers. Nature 568, 551–556 (2019).
Russo, M. et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 366, 1473–1480 (2019).
Isogawa, A., Fuchs, R. P. & Fujii, S. Chromatin pull-down methodology based on DNA triple helix formation. Methods Mol. Biol. 2119, 183–199 (2020).
Schaub, J. M., Zhang, H., Soniat, M. M. & Finkelstein, I. J. Assessing protein dynamics on low-complexity single-stranded DNA curtains. Langmuir 34, 14882–14890 (2018).
Collins, B. E., Ye, L. F., Duzdevich, D. & Greene, E. C. DNA curtains: novel tools for imaging protein-nucleic acid interactions at the single-molecule level. Methods Cell Biol. 123, 217–234 (2014).
Nagel, Z. D. et al. Towards precision prevention: technologies for identifying healthy individuals with high risk of disease. Mutat. Res. 800–802, 14–28 (2017).
Azqueta, A. et al. DNA repair as a human biomonitoring tool: comet assay approaches. Mutat. Res. 781, 71–87 (2019).
Gajski, G. et al. The comet assay in animal models: from bugs to whales (Part 2, Vertebrates). Mutat. Res. 781, 130–164 (2019).
Ge, J. et al. CometChip: a high-throughput 96-well platform for measuring DNA damage in microarrayed human cells. J. Vis. Exp. 92, e50607 (2014).
Muruzabal, D. et al. Novel approach for the detection of alkylated bases using the enzyme-modified comet assay. Toxicol. Lett. 330, 108–117 (2020).
Ngo, L. P. et al. Sensitive CometChip assay for screening potentially carcinogenic DNA adducts by trapping DNA repair intermediates. Nucleic Acids Res. 48, e13 (2020).
Li, J. et al. DNA Repair Molecular Beacon assay: a platform for real-time functional analysis of cellular DNA repair capacity. Oncotarget 9, 31719–31743 (2018).
Forestier, A., Sarrazy, F., Caillat, S., Vandenbrouck, Y. & Sauvaigo, S. Functional DNA repair signature of cancer cell lines exposed to a set of cytotoxic anticancer drugs using a multiplexed enzymatic repair assay on biochip. PLoS ONE 7, e51754 (2012).
Pons, B. et al. Age-associated modifications of Base Excision Repair activities in human skin fibroblast extracts. Mech. Ageing Dev. 131, 661–665 (2010).
Shen, J. C., Fox, E. J., Ahn, E. H. & Loeb, L. A. A rapid assay for measuring nucleotide excision repair by oligonucleotide retrieval. Sci. Rep. 4, 4894 (2014).
Golato, T. et al. Development of a cell-based assay for measuring base excision repair responses. Sci. Rep. 7, 13007 (2017).
Luria, S. E. Reactivation of ultraviolet-inactivated bacteriophage particles inside double-infected host cells. J. Bacteriol. 54, 79 (1947).
Protic-Sabljic, M. & Kraemer, K. H. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc. Natl Acad. Sci. USA 82, 6622–6626 (1985).
Tsien, R. Y. Constructing and exploiting the fluorescent protein paintbox (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 48, 5612–5626 (2009).
Ito, S. et al. Fluorescence detection of DNA mismatch repair in human cells. Sci. Rep. 8, 12181 (2018).
Shuen, A. Y. et al. Functional repair assay for the diagnosis of constitutional mismatch repair deficiency from non-neoplastic tissue. J. Clin. Oncol. 37, 461–470 (2019).
Drost, M. et al. A functional assay-based procedure to classify mismatch repair gene variants in Lynch syndrome. Genet. Med. 21, 1486–1496 (2019).
Hempelmann, J. A., Scroggins, S. M., Pritchard, C. C. & Salipante, S. J. MSIplus for integrated colorectal cancer molecular testing by next-generation sequencing. J. Mol. Diagn. 17, 705–714 (2015).
Salipante, S. J., Scroggins, S. M., Hampel, H. L., Turner, E. H. & Pritchard, C. C. Microsatellite instability detection by next generation sequencing. Clin. Chem. 60, 1192–1199 (2014).
Boland, C. R. et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 58, 5248–5257 (1998).
Huerfano, S., Ryabchenko, B. & Forstová, J. Nucleofection of expression vectors induces a robust interferon response and inhibition of cell proliferation. DNA Cell Biol. 32, 467–479 (2013).
Fu, Y. et al. Inhibition of cGAS-mediated interferon response facilitates transgene expression. iScience 23, 101026 (2020).
Nakagawa, T., Bulger, M., Muramatsu, M. & Ito, T. Multistep chromatin assembly on supercoiled plasmid DNA by nucleosome assembly protein-1 and ATP-utilizing chromatin assembly and remodeling factor. J. Biol. Chem. 276, 27384–27391 (2001).
Shaul, O. How introns enhance gene expression. Int. J. Biochem. Cell Biol 91, 145–155 (2017).
Kozak, M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15, 8125–8148 (1987).
Roy, S. & Schreiber, E. Detecting and quantifying low level gene variants in Sanger sequencing traces using the ab1 peak reporter tool. J. Biomol. Tech 25, S13–S14 (2014).
Felgner, P. L. et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl Acad. Sci. USA 84, 7413–7417 (1987).
Sun, M., Bernard, L. P., Dibona, V. L., Wu, Q. & Zhang, H. Calcium phosphate transfection of primary hippocampal neurons. J. Vis. Exp. 81, e50808 (2013).
Shapiro, H. Practical Flow Cytometry 4th edn (Wiley, 2003).
Kiziltepe, T. et al. Delineation of the chemical pathways underlying nitric oxide-induced homologous recombination in mammalian cells. Chem. Biol. 12, 357–369 (2005).
Perfetto, S. P., Ambrozak, D., Nguyen, R., Chattopadhyay, P. & Roederer, M. Quality assurance for polychromatic flow cytometry. Nat. Protoc. 1, 1522–1530 (2006).
Fong, Y. W., Cattoglio, C. & Tjian, R. The intertwined roles of transcription and repair proteins. Mol. Cell 52, 291–302 (2013).
Acknowledgements
This work was supported by National Institutes of Health grants 1U01ES029520, P30ES000002 and 5P01CA092584.
Author information
Authors and Affiliations
Contributions
C.G.P., T.J.P. and D.J.L. prepared samples, designed and conducted experiments and developed the method. They were supervised by Z.D.N. All authors contributed to the writing and editing of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Bennet van Houten and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Nagel, Z. D. et al. Proc. Natl Acad. Sci. USA 111, E1823–E1832 (2014): https://doi.org/10.1073/pnas.1401182111
Chaim, I. A. et al. Proc. Natl Acad. Sci. USA 114, E10379–E10388 (2017): https://doi.org/10.1073/pnas.1712032114
Nagel, Z. D. et al. Cancer Res. 77, 198–206 (2017): https://doi.org/10.1158/0008-5472.can-16-1151
Extended data
Extended Data Fig. 1 Gel electrophoretic analysis and flow cytometric validation of GFP_Hx, mPlum_A-8oxoG and mOrange_8oxoG-C reporter plasmids.
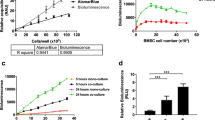
a, Analytical digest and flow cytometric validation of GFP_Hx plasmid. Lane 1: NEB 1-kb MW ladder; lane 2: pMax_GFP_C289T ccDNA; lane 3: HIA overnight extension reaction for GFP_Hx; lane 4: pMax_GFP_C289T ccDNA; lane 5: pMax_GFP_C289T after a 45-min ApaLI digestion at 37 °C, which cleaves two restriction sites, resulting in two linear DNA fragments; lane 6: GFP_Hx ccDNA after T5 Exo and PEG purification steps; and lane 7: GFP_Hx after a 45-min ApaLI digestion at 37 °C, in which the Hx lesion blocks ApaLI cleavage of one restriction site, leaving a single linearized fragment. At right: flow cytometric quantitation of percent reporter expression and normalized relative reporter expression in WT HAP cells compared to MPG−/− HAP cells. b, Analytical digestion and flow cytometric validation of mPlum_A-8oxoG plasmid. Lane 1: HIA overnight extension reaction for mPlum_A-8oxoG; lane 2: pMax_mPlum ccDNA; lane 3: pMax_mPlum after a 45-min Fpg endonuclease digestion at 37 °C; lane 4: mPlum_A-8oxoG after T5 Exo and PEG purification steps; lane 5: mPlum_A-8oxoG after a 45-min Fpg endonuclease digestion at 37 °C, resulting in plasmid nicking and upward mobility shift. At right: flow cytometric quantitation of percent reporter expression and normalized relative reporter expression in WT HAP cells compared to MUTYH−/− HAP cells. c, Analytical digestion and flow cytometric validation of mOrange_8oxoG-C plasmid. Lane 1: NEB 1-kb MW ladder; lane 2: pMax_mOrange_A215C ssDNA; lane 3: pMax_mOrange_A215C ocDNA; lane 4: pMax_mOrange_A215C ccDNA; lane 5: pMax_mOrange_A215C after a 45-min Fpg endonuclease digestion at 37 °C; lane 6: mOrange_8oxoG-C ccDNA; and lane 7: mOrange_8oxoG-C after a 45-min Fpg endonuclease digestion at 37 °C, which introduces a nick at the 8oxoG lesion, resulting in upward mobility shift. At right: flow cytometric quantitation of percent reporter expression and normalized relative reporter expression in WT MEF cells compared to OGG1−/− MEF cells. Error bars represent s.e.m. from three to four biological replicates; differences of statistical significance (*, P < 0.05; ***, P < 0.005; ****, P < 0.0001) were determined by unpaired two-tailed t test.
Extended Data Fig. 2 Gel electrophoretic analysis and flow cytometric validation of mPlum_O6-MeG and BFP_U reporter plasmids.
a, Analytical digestion and flow cytometric validation of mPlum_O6-MeG plasmid. Lane 1: NEB 1-kb MW ladder; lane 2: pMax_mPlum_C207G/T208C ccDNA; lane 3: pMax_mPlum_C207G/T208C after a 45-min PspOMI digestion at 37 °C, resulting in linear pMax_mPlum_C207G/T208C starting plasmid; lane 4: mPlum_O6-MeG plasmid after T5 Exo and PEG purification steps; lane 5: mPlum_O6-MeG after a 45-min PspOMI digestion at 37 °C, in which the O6 group on the guanine blocks linearization by PspOMI, leaving predominantly ccDNA product (note: upon extended digestion or when excess enzyme is present, some linearized DNA will result). At right: flow cytometric quantitation of percent reporter expression and normalized relative reporter expression in MGMT-deficient TK6 cells compared to TK6 cells complimented with stable MGMT expression. b, Analytical digestion and flow cytometric validation of BFP_U plasmid. Lane 1: NEB 1-kb MW ladder; lane 2: pMax_BFP_A191G ccDNA; lane 3: pMax_BFP_A191G ocDNA; lane 4: pMax_BFP_A191G after a 5-min UDG digestion at 37 °C, followed by a 30-min APE1 digestion at 37 °C; lane 5: BFP_U after a 5-min UDG digestion at 37 °C, followed by a 30-min APE1 digestion at 37 °C, resulting in UDG excising the incorporated uracil, followed by APE1 nicking the abasic site, resulting in an upward gel mobility shift; lane 6: BFP_U plasmid after T5 Exo and PEG purification steps. At right: Flow cytometric quantitation of percent reporter expression and normalized relative reporter expression in WT MEF cells compared to UNG−/− MEF cells. Error bars represent s.e.m. from three to four biological replicates; differences of statistical significance (*, P < 0.05; **, P < 0.005; ***, P < 0.005) were determined by unpaired two-tailed t test.
Extended Data Fig. 3 Gel electrophoretic analysis and flow cytometric validation of mOrange_GG plasmid.
a, Gel electrophoretic analysis of mOrange_GG plasmid. Lane 1: NEB 1-kb MW ladder; lane 2: PMax_mOrange_G299C ccDNA; lane 3: HIA overnight extension reaction for mOrange_GG; lane 4: mOrange_GG after a 3-h digestion with T5 Exo; lane 5: mOrange_GG after PEG precipitation step; and lane 6: final mOrange_GG ccDNA after T5 Exo and PEG purification steps. b, Flow cytometric quantitation of percent reporter expression and normalized relative reporter expression in TK6 cells compared to MMR-deficient MT1 lymphoblastoid cells. Error bars represent s.e.m. from three to four biological replicates; differences of statistical significance (***, P < 0.005) were determined by unpaired two-tailed t test.
Supplementary information
Source data
Source Data Fig. 7
Raw data used in column plots.
Source Data Extended Data Fig. 1
Raw data used in column plots.
Source Data Extended Data Fig. 2
Raw data used in column plots.
Source Data Extended Data Fig. 3
Raw data used in column plots.
Source Data Extended Data Fig. 1
Unprocessed gel images.
Source Data Extended Data Fig. 2
Unprocessed gel images.
Source Data Extended Data Fig. 3
Unprocessed gel images.
Rights and permissions
About this article
Cite this article
Piett, C.G., Pecen, T.J., Laverty, D.J. et al. Large-scale preparation of fluorescence multiplex host cell reactivation (FM-HCR) reporters. Nat Protoc 16, 4265–4298 (2021). https://doi.org/10.1038/s41596-021-00577-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00577-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.