Abstract
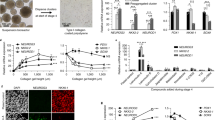
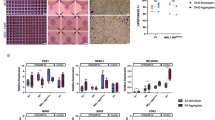
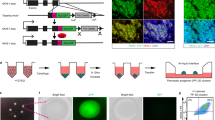
We detail a six-stage planar differentiation methodology for generating human pluripotent stem cell–derived pancreatic β cells (SC-β cells) that secrete high amounts of insulin in response to glucose stimulation. This protocol first induces definitive endoderm by treatment with Activin A and CHIR99021, then generates PDX1+/NKX6-1+ pancreatic progenitors through the timed application of keratinocyte growth factor, SANT1, TPPB, LDN193189 and retinoic acid. Endocrine induction and subsequent SC-β-cell specification is achieved with a cocktail consisting of the cytoskeletal depolymerizing compound latrunculin A combined with XXI, T3, ALK5 inhibitor II, SANT1 and retinoic acid. The resulting SC-β cells and other endocrine cell types can then be aggregated into islet-like clusters for analysis and transplantation. This differentiation methodology takes ~34 d to generate functional SC-β cells, plus an additional 1–2 weeks for initial stem cell expansion and final cell assessment. This protocol builds upon a large body of previous work for generating β-like cells. In this iteration, we have eliminated the need for 3D culture during endocrine induction, allowing for the generation of highly functional SC-β cells to be done entirely on tissue culture polystyrene. This change simplifies the differentiation methodology, requiring only basic stem cell culture experience as well as familiarity with assessment techniques common in biology laboratories. In addition to expanding protocol accessibility and simplifying SC-β-cell generation, we demonstrate that this planar methodology is amenable for differentiating SC-β cells from a wide variety of cell lines from various sources, broadening its applicability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with this paper. Any other data are available from the corresponding author upon reasonable request.
References
Brennan, D. C., Kopetskie, H. A. & Sayre, P. H. Long-term follow-up of the Edmonton protocol of islet transplantation in the United States. Am. J. Transpl. 16, 509–517 (2016).
Shapiro, J. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238 (2000).
D’Amour, K. A. et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 (2005).
D’Amour, K. A. et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401 (2006).
Nostro, M. C. et al. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 138, 861–871 (2011).
Kunisada, Y., Tsubooka-Yamazoe, N., Shoji, M. & Hosoya, M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 8, 274–284 (2012).
Borowiak, M. et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell 4, 348–358 (2009).
Sneddon, J. B., Borowiak, M. & Melton, D. A. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature 491, 765–768 (2012).
Mahaddalkar, P. U. et al. Generation of pancreatic β cells from CD177+ anterior definitive endoderm. Nat. Biotechnol. 38, 1061–1072 (2020).
Xu, X., Browning, V. L. & Odorico, J. S. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech. Dev. 128, 412–427 (2011).
Nostro, M. C. & Keller, G. Generation of beta cells from human pluripotent stem cells: Potential for regenerative medicine. Semin. Cell Dev. Biol. 23, 701–710 (2012).
Kroon, E. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443–452 (2008).
Rezania, A. et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 61, 2016–2029 (2012).
Bruin, J. E. et al. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 56, 1987–1998 (2013).
Bruin, J. E. et al. Accelerated maturation of human stem cell-derived pancreatic progenitor cells into insulin-secreting cells in immunodeficient rats relative to mice. Stem Cell Rep. 5, 1081–1096 (2015).
Rezania, A. et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 31, 2432–2442 (2013).
Kelly, O. G. et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat. Biotechnol. 29, 750–756 (2011).
Schaffer, A. E., Freude, K. K., Nelson, S. B. & Sander, M. Nkx6 transcription factors and Ptf1a function as antagonistic lineage determinants in multipotent pancreatic progenitors. Dev. Cell 18, 1022–1029 (2010).
Nelson, S. B., Schaffer, A. E. & Sander, M. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development 134, 2491–2500 (2007).
Nostro, M. C. et al. Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Rep. 4, 591–604 (2015).
Veres, A. et al. Charting cellular identity during human in vitro β -cell differentiation. Nature 569, 368–373 (2019).
Sharon, N. et al. Wnt signaling separates the progenitor and endocrine compartments during pancreas development. Cell Rep. 27, 2281–2291 (2019).
Johansson, K. A. et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell 12, 457–465 (2007).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Rezania, A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133 (2014).
Russ, H. A. et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 34, 1759–1772 (2015).
Nair, G. G. et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 21, 263–274 (2019).
Sui, L. et al. β-Cell replacement in mice using human type 1 diabetes nuclear transfer embryonic stem cells. Diabetes 67, 26–35 (2018).
Alvarez-Dominguez, J. R. et al. Circadian entrainment triggers maturation of human in vitro islets. Cell Stem Cell 26, 108–122.e10 (2020).
Zhou, X. et al. LIN28B Impairs the transition of hESC-derived β cells from the juvenile to adult state. Stem Cell Rep. 14, 9–20 (2020).
Cogger, K. F. et al. Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors. Nat. Commun. 8, 331 (2017).
Johannesson, B., Sui, L., Freytes, D. O., Creusot, R. J. & Egli, D. Toward beta cell replacement for diabetes. EMBO J. 34, 841–855 (2015).
Sneddon, J. B. et al. Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell 22, 810–823 (2018).
Millman, J. R. & Pagliuca, F. W. Autologous pluripotent stem cell-derived β-like cells for diabetes cellular therapy. Diabetes 66, 1111–1120 (2017).
Nair, G. G., Tzanakakis, E. S. & Hebrok, M. Emerging routes to the generation of functional β-cells for diabetes mellitus cell therapy. Nat. Rev. Endocrinol. 16, 506–518 (2020).
Latres, E., Finan, D. A., Greenstein, J. L., Kowalski, A. & Kieffer, T. J. Navigating two roads to glucose normalization in diabetes: automated insulin delivery devices and cell therapy. Cell Metab. 29, 545–563 (2019).
Johnson, J. D. The quest to make fully functional human pancreatic beta cells from embryonic stem cells: climbing a mountain in the clouds. Diabetologia 59, 2047–2057 (2016).
Tremmel, D. M., Mitchell, S. A., Sackett, S. D. & Odorico, J. S. Mimicking nature-made beta cells: recent advances towards stem cell-derived islets. Curr. Opin. Organ Transplant. 24, 574–581 (2019).
Velazco-Cruz, L., Goedegebuure, M. M. & Millman, J. R. Advances toward engineering functionally mature human pluripotent stem cell-derived β cells. Front. Bioeng. Biotechnol. 8, 786 (2020).
Velazco-Cruz, L. et al. Acquisition of dynamic function in human stem cell-derived β cells. Stem Cell Rep. 12, 351–365 (2019).
Hogrebe, N. J., Augsornworawat, P., Maxwell, K. G., Velazco-Cruz, L. & Millman, J. R. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 38, 460–470 (2020).
Maxwell, K. G. et al. Gene-edited human stem cell–derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med. 12, eaax9106 (2020).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Kilian, K. A., Bugarija, B., Lahn, B. T. & Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl Acad. Sci. 107, 4872–4877 (2010).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004).
Mamidi, A. et al. Mechanosignalling via integrins directs fate decisions of pancreatic progenitors. Nature 564, 114–118 (2018).
Rosado-Olivieri, E. A., Anderson, K., Kenty, J. H. & Melton, D. A. YAP inhibition enhances the differentiation of functional stem cell-derived insulin-producing β cells. Nat. Commun. 10, 1464 (2019).
Cebola, I. et al. TEAD and YAP regulate the enhancer network of human embryonic pancreatic progenitors. Nat. Cell Biol. 17, 615–626 (2015).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Slaoui, M. & Fiette, L. Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol. Biol. 691, 69–82 (2011).
Augsornworawat, P., Maxwell, K. G., Velazco-Cruz, L. & Millman, J. R. Single-cell transcriptome profiling reveals β cell maturation in stem cell-derived islets after transplantation. Cell Rep. 32, 108067 (2020).
Acknowledgements
This work was supported by the JDRF Career Development Award (5-CDA-2017-391-A-N), the National Institutes of Health (NIH) (R01DK114233, R01DK127497) and startup funds from the Washington University School of Medicine Department of Medicine. N.J.H. was supported by the JDRF (3-APF-2020-930-A-N). K.G.M. was supported by the NIH (T32DK108742). Microscopy was performed through the Washington University Center for Cellular Imaging, which is supported by the Washington University School of Medicine, the Children’s Discovery Institute (CDI-CORE-2015-505) and the Foundation for Barnes-Jewish Hospital (3770). Microscopy analysis was supported by the Washington University Diabetes Research Center (P30DK020579).
Author information
Authors and Affiliations
Contributions
N.J.H., K.G.M. and P.A. collected data for all experiments. N.J.H., K.G.M., P.A. and J.R.M. designed the experiments, wrote the manuscript and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
N.J.H., K.G.M., P.A., and J.R.M. are inventors on patents and patent applications related to SC-β cells. J.R.M. is a consultant for Sana Biotechnology. K.G.M., P.A. and J.R.M. are cofounders of Salentra Biosciences.
Additional information
Peer review information Nature Protocols thanks Heiko Lickert and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Hogrebe, N. et al. Nat. Biotechnol. 38, 460–470 (2020): https://doi.org/10.1038/s41587-020-0430-6
Maxwell, K. G. et al. Sci. Transl. Med. 12, eaax9106 (2020): https://doi.org/10.1126/scitranslmed.aax9106
Augsornworawat, P. et al. Cell Reports 32, 108067 (2020): https://doi.org/10.1016/j.celrep.2020.108067
Supplementary information
Supplementary Information
Supplementary Figs. 1–7.
Source data
Source Data Fig. 5
Source data and example calculations for Fig. 5.
Source Data Fig. 6
Source data and example calculations for Fig. 6.
Rights and permissions
About this article
Cite this article
Hogrebe, N.J., Maxwell, K.G., Augsornworawat, P. et al. Generation of insulin-producing pancreatic β cells from multiple human stem cell lines. Nat Protoc 16, 4109–4143 (2021). https://doi.org/10.1038/s41596-021-00560-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00560-y
This article is cited by
-
Comparative and integrative single cell analysis reveals new insights into the transcriptional immaturity of stem cell-derived β cells
BMC Genomics (2024)
-
Standardized production of hPSC-derived cardiomyocyte aggregates in stirred spinner flasks
Nature Protocols (2024)
-
Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques
Nature Biotechnology (2024)
-
Islets in the body are never flat: transitioning from two-dimensional (2D) monolayer culture to three-dimensional (3D) spheroid for better efficiency in the generation of functional hPSC-derived pancreatic β cells in vitro
Cell Communication and Signaling (2023)
-
Suspension culture improves iPSC expansion and pluripotency phenotype
Stem Cell Research & Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.