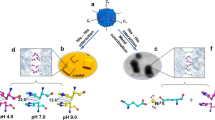
Abstract
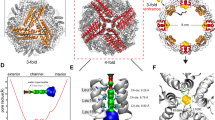
The self-assembly of proteins into sophisticated multicomponent assemblies is a hallmark of all living systems and has spawned extensive efforts in the construction of novel synthetic protein architectures with emergent functional properties. Protein assemblies in nature are formed via selective association of multiple protein surfaces through intricate noncovalent protein–protein interactions, a challenging task to accurately replicate in the de novo design of multiprotein systems. In this protocol, we describe the application of metal-coordinating hydroxamate (HA) motifs to direct the metal-mediated assembly of polyhedral protein architectures and 3D crystalline protein–metal–organic frameworks (protein-MOFs). This strategy has been implemented using an asymmetric cytochrome cb562 monomer through selective, concurrent association of Fe3+ and Zn2+ ions to form polyhedral cages. Furthermore, the use of ditopic HA linkers as bridging ligands with metal-binding protein nodes has allowed the construction of crystalline 3D protein-MOF lattices. The protocol is divided into two major sections: (1) the development of a Cys-reactive HA molecule for protein derivatization and self-assembly of protein–HA conjugates into polyhedral cages and (2) the synthesis of ditopic HA bridging ligands for the construction of ferritin-based protein-MOFs using symmetric metal-binding protein nodes. Protein cages are analyzed using analytical ultracentrifugation, transmission electron microscopy and single-crystal X-ray diffraction techniques. HA-mediated protein-MOFs are formed in sitting-drop vapor diffusion crystallization trays and are probed via single-crystal X-ray diffraction and multi-crystal small-angle X-ray scattering measurements. Ligand synthesis, construction of HA-mediated assemblies, and post-assembly analysis as described in this protocol can be performed by a graduate-level researcher within 6 weeks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The principal data supporting the findings of this work are available within the figures and the Supplementary Information. Additional data that support the findings of this study are available from the corresponding author on request.
References
Marsh, J. A. & Teichmann, S. A. Structure, dynamics, assembly, and evolution of protein complexes. Annu. Rev. Biochem. 84, 551–575 (2015).
Barber, J. Photosystem II: the engine of life. Q. Rev. Biophys. 36, 71–89 (2003).
Eitoku, M., Sato, L., Senda, T. & Horikoshi, M. Histone chaperones: 30 years from isolation to elucidation of the mechanisms of nucleosome assembly and disassembly. Cell. Mol. Life Sci. 65, 414–444 (2008).
Yeates, T. O. Geometric principles for designing highly symmetric self-assembling protein nanomaterials. Annu. Rev. Biophys. 46, 23–42 (2017).
Bai, Y., Luo, Q. & Liu, J. Protein self-assembly via supramolecular strategies. Chem. Soc. Rev. 45, 2756–2767 (2016).
Hamley, I. W. Protein assemblies: nature-inspired and designed nanostructures. Biomacromolecules 20, 1829–1848 (2019).
Churchfield, L. A. & Tezcan, F. A. Design and construction of functional supramolecular metalloprotein assemblies. Acc. Chem. Res. 52, 345–355 (2019).
Seeman, N. C. & Sleiman, H. F. DNA nanotechnology. Nat. Rev. Mater. 3, 17068 (2017).
Cannon, K. A., Ochoa, J. M. & Yeates, T. O. High-symmetry protein assemblies: patterns and emerging applications. Curr. Opin. Struc. Biol. 55, 77–84 (2019).
Golub, E. et al. Constructing protein polyhedra via orthogonal chemical interactions. Nature 578, 172–176 (2020).
Sontz, P. A., Bailey, J. B., Ahn, S. & Tezcan, F. A. A metal organic framework with spherical protein nodes: rational chemical design of 3D protein crystals. J. Am. Chem. Soc. 137, 11598–11601 (2015).
Brodin, J. D. et al. Metal-directed, chemically tunable assembly of one-, two- and three-dimensional crystalline protein arrays. Nat. Chem. 4, 375–382 (2012).
Song, W. J. & Tezcan, F. A. A designed supramolecular protein assembly with in vivo enzymatic activity. Science 346, 1525–1528 (2014).
Churchfield, L. A., Medina-Morales, A., Brodin, J. D., Perez, A. & Tezcan, F. A. De novo design of an allosteric metalloprotein assembly with strained disulfide bonds. J. Am. Chem. Soc. 138, 13163–13166 (2016).
Wong, G. B., Kappel, M. J., Raymond, K. N., Matzanke, B. & Winkelmann, G. Coordination chemistry of microbial iron transport compounds. 24. Characterization of coprogen and ferricrocin, two ferric hydroxamate siderophores. J. Am. Chem. Soc. 105, 810–815 (1983).
Crumbliss, A. L. Iron bioavailability and the coordination chemistry of hydroxamic acids. Coord. Chem. Rev. 105, 155–179 (1990).
Bailey, J. B., Zhang, L., Chiong, J. A., Ahn, S. & Tezcan, F. A. Synthetic modularity of protein–metal–organic frameworks. J. Am. Chem. Soc. 139, 8160–8166 (2017).
Bailey, J. B. & Tezcan, F. A. Tunable and cooperative thermomechanical properties of protein–metal–organic frameworks. J. Am. Chem. Soc. 142, 17265–17270 (2020).
Padilla, J. E., Colovos, C. & Yeates, T. O. Nanohedra: using symmetry to design self assembling protein cages, layers, crystals, and filaments. Proc. Natl Acad. Sci. USA 98, 2217–2221 (2001).
Lai, Y.-T., Cascio, D. & Yeates, T. O. Structure of a 16-nm cage designed by using protein oligomers. Science 336, 1129 (2012).
Lai, Y.-T. et al. Structure of a designed protein cage that self-assembles into a highly porous cube. Nat. Chem. 6, 1065–1071 (2014).
Cannon, K. A., Nguyen, V. N., Morgan, C. & Yeates, T. O. Design and characterization of an icosahedral protein cage formed by a double-fusion protein containing three distinct symmetry elements. ACS Synth. Biol. 9, 517–524 (2020).
Cristie-David, A. S. et al. Coiled-coil-mediated assembly of an icosahedral protein cage with extremely high thermal and chemical stability. J. Am. Chem. Soc. 141, 9207–9216 (2019).
Sinclair, J. C., Davies, K. M., Venien-Bryan, C. & Noble, M. E. M. Generation of protein lattices by fusing proteins with matching rotational symmetry. Nat. Nanotechnol. 6, 558–562 (2011).
Hsia, Y. et al. Design of a hyperstable 60-subunit protein dodecahedron. Nature 535, 136–139 (2016).
Bale, J. B. et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science 353, 389–394 (2016).
Gonen, S., DiMaio, F., Gonen, T. & Baker, D. Design of ordered two-dimensional arrays mediated by noncovalent protein-protein interfaces. Science 348, 1365–1368 (2015).
Suzuki, Y. et al. Self-assembly of coherently dynamic, auxetic, two-dimensional protein crystals. Nature 533, 369–373 (2016).
Alberstein, R., Suzuki, Y., Paesani, F. & Tezcan, F. A. Engineering the entropy-driven free-energy landscape of a dynamic nanoporous protein assembly. Nat. Chem. 10, 732–739 (2018).
Malay, A. D. et al. An ultra-stable gold-coordinated protein cage displaying reversible assembly. Nature 569, 438–442 (2019).
Cristie-David, A. S. & Marsh, E. N. G. Metal-dependent assembly of a protein nano-cage. Protein Sci. 28, 1620–1629 (2019).
Song, W. J., Sontz, P. A., Ambroggio, X. I. & Tezcan, F. A. Metals in protein-protein interfaces. Ann. Rev. Biophys. 43, 409–431 (2014).
Salgado, E. N., Faraone-Mennella, J. & Tezcan, F. A. Controlling protein-protein interactions through metal coordination: assembly of a 16-helix bundle protein. J. Am. Chem. Soc. 129, 13374–13375 (2007).
Salgado, E. N., Lewis, R. A., Mossin, S., Rheingold, A. L. & Tezcan, F. A. Control of protein oligomerization symmetry by metal coordination: C2 and C3 symmetrical assemblies through CuII and NiII coordination. Inorg. Chem. 48, 2726–2728 (2009).
Salgado, E. N. et al. Metal-templated design of protein interfaces. Proc. Natl Acad. Sci. USA 107, 1827–1832 (2010).
Salgado, E. N., Radford, R. J. & Tezcan, F. A. Metal-directed protein self-assembly. Acc. Chem. Res. 43, 661–672 (2010).
Brodin, J. D. et al. Evolution of metal selectivity in templated protein interfaces. J. Am. Chem. Soc. 132, 8610–8617 (2010).
Medina-Morales, A., Perez, A., Brodin, J. D. & Tezcan, F. A. In vitro and cellular self-assembly of a Zn-binding protein cryptand via templated disulfide bonds. J. Am. Chem. Soc. 135, 12013–12022 (2013).
Brodin, J. D., Smith, S. J., Carr, J. R. & Tezcan, F. A. Designed, helical protein nanotubes with variable diameters from a single building block. J. Am. Chem. Soc. 137, 10468–10471 (2015).
Song, W. J., Yu, J. & Tezcan, F. A. Importance of scaffold flexibility/rigidity in the design and directed evolution of artificial metallo-β-lactamases. J. Am. Chem. Soc. 139, 16772–16779 (2017).
Churchfield, L. A., Alberstein, R. G., Williamson, L. M. & Tezcan, F. A. Determining the structural and energetic basis of allostery in a de novo designed metalloprotein assembly. J. Am. Chem. Soc. 140, 10043–10053 (2018).
Edwardson, T. G. W. & Hilvert, D. Virus-inspired function in engineered protein cages. J. Am. Chem. Soc. 141, 9432–9443 (2019).
Heddle, J. G., Chakraborti, S. & Iwasaki, K. Natural and artificial protein cages: design, structure and therapeutic applications. Curr. Opin. Struct. Biol. 43, 148–155 (2017).
Margolin, A. L. & Navia, M. A. Protein crystals as novel catalytic materials. Angew. Chem. Int. Ed. 40, 2205–2222 (2001).
Abe, S. et al. Design of enzyme-encapsulated protein containers by in vivo crystal engineering. Adv. Mater. 27, 7951–7956 (2015).
McPherson, A. & Gavira, J. A. Introduction to protein crystallization. Acta Crystallogr. F. Struct. Biol. Commun. 70, 2–20 (2014).
Johnson, J. E. & Speir, J. A. Quasi-equivalent viruses: a paradigm for protein assemblies. J. Mol. Biol. 269, 665–675 (1997).
Mateu, M. G. Assembly, stability and dynamics of virus capsids. Arch. Biochem. Biophys. 531, 65–79 (2013).
Radford, R. J., Nguyen, P. C., Ditri, T. B., Figueroa, J. S. & Tezcan, F. A. Controlled protein dimerization through hybrid coordination motifs. Inorg. Chem. 49, 4362–4369 (2010).
Radford, R. J., Nguyen, P. C. & Tezcan, F. A. Modular and versatile hybrid coordination motifs on alpha-helical protein surfaces. Inorg. Chem. 2010, 7106–7115 (2010).
Yang, M. & Song, W. J. Diverse protein assembly driven by metal and chelating amino acids with selectivity and tunability. Nat. Commun. 10, 5545 (2019).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Baldwin, A. D. & Kiick, K. L. Tunable degradation of maleimide–thiol adducts in reducing environments. Bioconjug. Chem. 22, 1946–1953 (2011).
Toussaint, L., Bertrand, L., Hue, L., Crichton, R. R. & Declercq, J.-P. High-resolution X-ray structures of human apoferritin H-chain mutants correlated with their activity and metal-binding sites. J. Mol. Biol. 365, 440–452 (2007).
Kanekiyo, M., Ellis, D. & King, N. P. New vaccine design and delivery technologies. J. Infect. Dis. 219, S88–S96 (2019).
Butterfield, G. L. et al. Evolution of a designed protein assembly encapsulating its own RNA genome. Nature 552, 415–420 (2017).
Uchida, M. et al. Modular self-assembly of protein cage lattices for multistep catalysis. ACS Nano 12, 942–953 (2018).
MaHam, A., Tang, Z. W., Wu, H., Wang, J. & Lin, Y. H. Protein-based nanomedicine platforms for drug delivery. Small 5, 1706–1721 (2009).
Dreschel, H. & Winkelmann, G. Iron chelation and siderophores. in Transition Metals in Microbial Metabolism (eds Winkelmann, G. & Carrano, C. J.) 1–51 (Harwood Academic Publishers, 1997).
Kaes, C., Katz, A. & Hosseini, M. W. Bipyridine: the most widely used ligand. A review of molecules comprising at least two 2,2′-bipyridine units. Chem. Rev. 100, 3553–3590 (2000).
Hofmeier, H. & Schubert, U. S. Recent developments in the supramolecular chemistry of terpyridine–metal complexes. Chem. Soc. Rev. 33, 373–399 (2004).
Jiang, P. & Guo, Z. Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors. Coord. Chem. Rev. 248, 205–229 (2004).
Xie, J., Liu, W. & Schultz, P. G. A genetically encoded bidentate, metal-binding amino acid. Angew. Chem. Int. Ed. Engl. 46, 9239–9242 (2007).
Lee, H. S., Spraggon, G., Schultz, P. G. & Wang, F. Genetic incorporation of a metal-ion chelating amino acid into proteins as a biophysical probe. J. Am. Chem. Soc. 131, 2481–2483 (2009).
Li, M. et al. Spectroscopic and crystallographic investigations of novel BODIPY-derived metal–organic frameworks. Inorg. Chem. 54, 1346–1353 (2015).
Bandara, H. M. D. & Burdette, S. C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 41, 1809–1825 (2012).
King, N. P. et al. Computational design of self-assembling protein nanomaterials with atomic level accuracy. Science 336, 1171–1174 (2012).
King, N. P. et al. Accurate design of co-assembling multi-component protein nanomaterials. Nature 510, 103–108 (2014).
Langan, R. A. et al. De novo design of bioactive protein switches. Nature 572, 205–210 (2019).
Yuan, Y., Tam, M. F., Simplaceanu, V. & Ho, C. New look at hemoglobin allostery. Chem. Rev. 115, 1702–1724 (2015).
McPherson, A. & Cudney, B. Optimization of crystallization conditions for biological macromolecules. Acta Cryst. F. Struct. Biol. Commun. 70, 1445–1467 (2014).
Goldschmidt, L., Cooper, D. R., Derewenda, Z. S. & Eisenberg, D. Toward rational protein crystallization: a web server for the design of crystallizable protein variants. Protein Sci. 16, 1569–1576 (2007).
Banatao, D. R. et al. An approach to crystallizing proteins by synthetic symmetrization. Proc. Natl Acad. Sci. USA 103, 16230–16235 (2006).
Künzle, M., Eckert, T. & Beck, T. Binary protein crystals for the assembly of inorganic nanoparticle superlattices. J. Am. Chem. Soc. 138, 12731–12734 (2016).
Chaudhury, S., Lyskov, S. & Gray, J. J. PyRosetta: a script-based interface for implementing molecular modeling algorithms using Rosetta. Bioinformatics 26, 689–691 (2010).
Ni, T. W. & Tezcan, F. A. Structural characterization of a microperoxidase inside a metal-directed protein cage. Angew. Chem. Int. Ed. 49, 7014–7018 (2010).
Michalak, K., Wicha, J. & Wójcik, J. Studies towards dynamic kinetic resolution of 4-hydroxy-2-methylcyclopent-2-en-1-one and its E-O-trityloxime. Tetrahedron 72, 4813–4820 (2016).
Faraone-Mennella, J., Tezcan, F. A., Gray, H. B. & Winkler, J. R. Stability and folding kinetics of structurally characterized cytochrome cb562. Biochemistry 45, 10504–10511 (2006).
Carrano, C. J. & Raymond, K. N. Coordination chemistry of microbial iron transport compounds. 10. Characterization of the complexes of rhodotorulic acid, a dihydroxamate siderophore. J. Am. Chem. Soc. 100, 5371–5374 (1978).
Pflugrath, J. W. Practical macromolecular cryocrystallography. Acta Crystallogr. F. Struct. Biol. Commun. 71, 622–642 (2015).
Bailey, J. B., Subramanian, R. H., Churchfield, L. A. & Tezcan, F. A. Metal-directed design of supramolecular protein assemblies. in Peptide, Protein and Enzyme Design (ed Pecoraro, V. L.) 223–250 (Academic Press, 2016).
Hem, J. D. & Skougstad, M. W. Coprecipitation effects in solutions containing ferrous, ferric, and cupric ions. USGS Report No. 1459E. https://doi.org/10.3133/wsp1459E (1960).
Lebowitz, J., Lewis, M. S. & Schuck, P. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci. 11, 2067–2079 (2002).
Cole, J. L., Lary, J. W., P. Moody, T. & Laue, T. M. Analytical ultracentrifugation: sedimentation velocity and sedimentation equilibrium. Methods Cell Biol. 84, 143–179 (2008).
Karplus, P. A. & Diederichs, K. Linking crystallographic model and data quality. Science 336, 1030–1033 (2012).
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D. 66, 133–144 (2010).
Kabsch, W. XDS. Acta Crystallogr. D. 66, 125–132 (2010).
Adams, P. D. et al. PHENIX—a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 210, 213–221 (2010).
Macrae, C. F. et al. Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Crystallogr. 53, 226–235 (2020).
Acknowledgements
We thank N. Avakyan and R. Alberstein for helpful discussions. This work was supported by the US Department of Energy (Division of Materials Sciences, Office of Basic Energy Sciences; DE-SC0003844; for protein-MOF work, including design, data collection and analysis as well as for protein-cage design and EM analyses) and by the National Science Foundation (Division of Materials Research; DMR-1602537 and DMR-2004558; crystallographic analyses of protein cages). R.H.S. was supported by the National Institute of Health Chemical Biology Interfaces Training Grant UC San Diego (T32GM112584).
Author information
Authors and Affiliations
Contributions
E.G. and F.A.T. conceived the protein-cage project. J.Z., J.A.C. and Y.L. synthesized HA ligands for the protein-cage project. E.G. and R.H.S. performed protein-cage experiments and data analysis. J.B.B. and F.A.T. conceived the protein-MOF project. J.B.B. synthesized ditopic HA ligands and performed protein-MOF experiments and data analysis. R.H.S. and F.A.T. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Christopher Snow and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Sontz, P. A. et al. J. Am. Chem. Soc. 137, 11598–11601 (2015): https://pubs.acs.org/doi/10.1021/jacs.5b07463
Bailey, J. B. et al. J. Am. Chem. Soc. 139, 8160–8166 (2017): https://pubs.acs.org/doi/10.1021/jacs.7b01202
Golub, E. et al. Nature 578, 172–176 (2020): https://www.nature.com/articles/s41586-019-1928-2
Bailey, J. B. et al. J. Am. Chem. Soc. 142, 17265–17270 (2020): https://pubs.acs.org/doi/10.1021/jacs.0c07835
Supplementary information
Supplementary Information
Supplementary Figs. 1–9.
Rights and permissions
About this article
Cite this article
Subramanian, R.H., Zhu, J., Bailey, J.B. et al. Design of metal-mediated protein assemblies via hydroxamic acid functionalities. Nat Protoc 16, 3264–3297 (2021). https://doi.org/10.1038/s41596-021-00535-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00535-z
This article is cited by
-
Supramolecular assembling systems of hemoproteins using chemical modifications
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2023)
-
Accurate computational design of three-dimensional protein crystals
Nature Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.