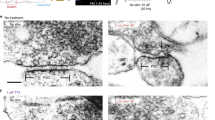
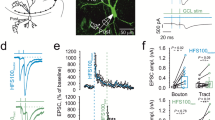
Abstract
Rigorous investigation of synaptic transmission requires analysis of unitary synaptic events by simultaneous recording from presynaptic terminals and postsynaptic target neurons. However, this has been achieved at only a limited number of model synapses, including the squid giant synapse and the mammalian calyx of Held. Cortical presynaptic terminals have been largely inaccessible to direct presynaptic recording, due to their small size. Here, we describe a protocol for improved subcellular patch-clamp recording in rat and mouse brain slices, with the synapse in a largely intact environment. Slice preparation takes ~2 h, recording ~3 h and post hoc morphological analysis 2 d. Single presynaptic hippocampal mossy fiber terminals are stimulated minimally invasively in the bouton-attached configuration, in which the cytoplasmic content remains unperturbed, or in the whole-bouton configuration, in which the cytoplasmic composition can be precisely controlled. Paired pre–postsynaptic recordings can be integrated with biocytin labeling and morphological analysis, allowing correlative investigation of synapse structure and function. Paired recordings can be obtained from mossy fiber terminals in slices from both rats and mice, implying applicability to genetically modified synapses. Paired recordings can also be performed together with axon tract stimulation or optogenetic activation, allowing comparison of unitary and compound synaptic events in the same target cell. Finally, paired recordings can be combined with spontaneous event analysis, permitting collection of miniature events generated at a single identified synapse. In conclusion, the subcellular patch-clamp techniques detailed here should facilitate analysis of biophysics, plasticity and circuit function of cortical synapses in the mammalian central nervous system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Original data are available in ref. 16 (https://doi.org/10.1016/j.neuron.2020.05.013). Further original data are stored in the scientific repository of the Institute of Science and Technology Austria and will be provided by the corresponding author upon reasonable request.
Code availability
Analysis software is available via GitHub (Stimfit version 0.15.8; https://github.com/neurodroid/stimfit). Further code for analysis of miniature EPSCs can be provided by the corresponding author upon reasonable request.
References
Adler, E. M., Augustine, G. J., Duffy, S. N. & Charlton, M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J. Neurosci. 11, 1496–1507 (1991).
Llinás, R. R. The Squid Giant Synapse: A Model for Chemical Transmission (Oxford University Press, 1999).
Forsythe, I. D. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J. Physiol. 479, 381–387 (1994).
von Gersdorff, H. & Borst, J. G. G. Short-term plasticity at the calyx of Held. Nat. Rev. Neurosci. 3, 53–64 (2002).
Neher, E. Some subtle lessons from the calyx of Held synapse. Biophys. J. 112, 215–223 (2017).
Borst, J. G. G. & Sakmann, B. Calcium influx and transmitter release in a fast CNS synapse. Nature 383, 431–434 (1996).
Vyleta, N. P. & Jonas, P. Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse. Science 343, 665–670 (2014).
Lindau, M. & Neher, E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflügers Arch. 411, 137–146 (1988).
von Gersdorff, H. & Mathews, G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature 367, 735–739 (1994).
Hallermann, S., Pawlu, C., Jonas, P. & Heckmann, M. A large pool of releasable vesicles in a cortical glutamatergic synapse. Proc. Natl. Acad. Sci. USA 100, 8975–8980 (2003).
Delvendahl, I., Vyleta, N. P., von Gersdorff, H. & Hallermann, S. Fast, temperature-sensitive and clathrin-independent endocytosis at central synapses. Neuron 90, 492–498 (2016).
Nicoll, R. A. & Schmitz, D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 6, 863–876 (2005).
Espinoza, C., Guzman, S. J., Zhang, X. & Jonas, P. Parvalbumin+ interneurons obey unique connectivity rules and establish a powerful lateral-inhibition microcircuit in dentate gyrus. Nat. Commun. 9, 4605 (2018).
Akaike, N., et al. Focal stimulation of single GABAergic presynaptic boutons on the rat hippocampal neuron. Neurosci. Res. 42, 187–195 (2002).
Vyleta, N. P., Borges-Merjane, C. & Jonas, P. Plasticity-dependent, full detonation at hippocampal mossy fiber-CA3 pyramidal neuron synapses. eLife 5, e17977 (2016).
Vandael, D., Borges-Merjane, C., Zhang, X. & Jonas, P. Short-term plasticity at hippocampal mossy fiber synapses is induced by natural activity patterns and associated with vesicle pool engram formation. Neuron 107, 509–521.e7 (2020).
Chicurel, M. E. & Harris, K. M. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J. Comp. Neurol. 325, 169–182 (1992).
Rollenhagen, A. et al. Structural determinants of transmission at large hippocampal mossy fiber synapses. J. Neurosci. 27, 10434–10444 (2007).
Borges-Merjane, C., Kim, O. & Jonas, P. Functional electron microscopy, ‘flash and freeze,’ of identified cortical synapses in acute brain slices. Neuron 105, 992–1006 (2020).
Rancz, E. A. et al. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature 450, 1245–1248 (2007).
Ritzau-Jost, A. et al. Ultrafast action potentials mediate kilohertz signaling at a central synapse. Neuron 84, 152–163 (2014).
Vivekananda, U. et al. Kv1.1 channelopathy abolishes presynaptic spike width modulation by subthreshold somatic depolarization. Proc. Natl. Acad. Sci. USA 114, 2395–2400 (2017).
Kawaguchi, S. Y. & Sakaba, T. Fast Ca2+ buffer-dependent reliable but plastic transmission at small CNS synapses revealed by direct bouton recording. Cell Rep. 21, 3338–3345 (2017).
Ritzau-Jost, A. et al. Large, stable spikes exhibit differential broadening in excitatory and inhibitory neocortical boutons. Cell Rep. 34, 108612 (2021).
Geiger, J. R. P. & Jonas, P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron 28, 927–939 (2000).
Bischofberger, J., et al. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat. Protoc. 1, 2075–2081 (2006).
Perkins, K. L. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J. Neurosci. Methods 154, 1–18 (2006).
Alcami, P., Franconville, R., Llano, I. & Marty, A. Measuring the firing rate of high-resistance neurons with cell-attached recording. J. Neurosci. 32, 3118–3130 (2012).
Engel, D. & Jonas, P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron 45, 405–417 (2005).
Dodt, H. U. & Zieglgänsberger, W. Infrared videomicroscopy: a new look at neuronal structure and function. Trends Neurosci. 17, 453–458 (1994).
Jonas, P., Major, G. & Sakmann, B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J. Physiol. 472, 615–663 (1993).
Lawrence, J. J., Grinspan, Z. M. & McBain, C. J. Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J. Physiol. 554, 175–193 (2004).
Kamiya, H., Shinozaki, H. & Yamamoto, C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J. Physiol. 493, 447–455 (1996).
Shigemoto, R. et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 17, 7503–7522 (1997).
Weisskopf, M. G. & Nicoll, R. A. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature 376, 256–259 (1995).
Ben-Simon, Y. et al. A combined optogenetic-knockdown strategy reveals a major role of tomosyn in mossy fiber synaptic plasticity. Cell Rep. 12, 396–404 (2015).
Mori, M., Abegg, M. H., Gähwiler, B. H. & Gerber, U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature 431, 453–456 (2004).
Bischofberger, J., Geiger, J. R. P. & Jonas, P. Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons. J. Neurosci. 22, 10593–10602 (2002).
Martinello, K., et al. The subthreshold-active Kv7 current regulates neurotransmission by limiting spike-induced Ca2+ influx in hippocampal mossy fiber synaptic terminals. Commun. Biol. 2, 145 (2019).
Alle, H. & Geiger, J. R. P. Combined analog and action potential coding in hippocampal mossy fibers. Science 311, 1290–1293 (2006).
Szabadics, J. & Soltesz, I. Functional specificity of mossy fiber innervation of GABAergic cells in the hippocampus. J. Neurosci. 29, 4239–4251 (2009).
Amaral, D. G. & Dent, J. A. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J. Comp. Neurol. 195, 51–86 (1981).
Bazigou, E. et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J. Clin. Invest. 121, 2984–2992 (2011).
Geiger, J. R. P. et al. Patch-clamp recording in brain slices with improved slicer technology. Pflügers Arch. 443, 491–501 (2002).
Edwards, F. A., Konnerth, A., Sakmann, B. & Takahashi, T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Arch. 414, 600–612 (1989).
Guzman, S. J., Schlögl, A. & Schmidt-Hieber, C. Stimfit: quantifying electrophysiological data with Python. Front. Neuroinform. 8, 16 (2014).
Williams, S. R. & Mitchell, S. J. Direct measurement of somatic voltage clamp errors in central neurons. Nat. Neurosci. 11, 790–798 (2008).
del Castillo, J. & Katz, B. Changes in end-plate activity produced by presynaptic polarization. J. Physiol. 124, 586–604 (1954).
Li, L., Bischofberger, J. & Jonas, P. Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J. Neurosci. 27, 13420–13429 (2007).
Pernía-Andrade, A. J. et al. A deconvolution-based method with high sensitivity and temporal resolution for detection of spontaneous synaptic currents in vitro and in vivo. Biophys. J. 103, 1429–1439 (2012).
Zhang, X., Schlögl, A., Vandael, D. & Jonas, P. MOD: a novel machine-learning optimal-filtering method for accurate and efficient detection of subthreshold synaptic events in vivo. J. Neurosci. Methods https://doi.org/10.1016/j.jneumeth.2021.109125 (2021).
Miyano, R., Miki, T. & Sakaba, T. Ca-dependence of synaptic vesicle exocytosis and endocytosis at the hippocampal mossy fibre terminal. J. Physiol. 597, 4373–4386 (2019).
Schneggenburger, R. & Neher, E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature 406, 889–893 (2000).
Bollmann, J. H., Sakmann, B. & Borst, J. G. G. Calcium sensitivity of glutamate release in a calyx-type terminal. Science 289, 953–957 (2000).
Midorikawa, M. & Sakaba, T. Kinetics of releasable synaptic vesicles and their plastic changes at hippocampal mossy fiber synapses. Neuron 96, 1033–1040 (2017).
Henze, D. A., McMahon, D. B., Harris, K. M. & Barrionuevo, G. Giant miniature EPSCs at the hippocampal mossy fiber to CA3 pyramidal cell synapse are monoquantal. J. Neurophysiol. 87, 15–29 (2002).
Salin, P. A., Scanziani, M., Malenka, R. C. & Nicoll, R. A. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc. Natl. Acad. Sci. USA 93, 13304–13309 (1996).
Jackman, S. L., Turecek, J., Belinsky, J. E. & Regehr, W. G. The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 529, 88–91 (2016).
Griffith, W. H. Voltage-clamp analysis of posttetanic potentiation of the mossy fiber to CA3 synapse in hippocampus. J. Neurophysiol. 63, 491–501 (1990).
Toth, K., Suares, G., Lawrence, J. J., Philips-Tansey, E. & McBain, C. J. Differential mechanisms of transmission at three types of mossy fiber synapse. J. Neurosci. 20, 8279–8289 (2000).
Acknowledgements
This project received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 692692 to P.J.) and the Fond zur Förderung der Wissenschaftlichen Forschung (Z 312-B27, Wittgenstein award to P.J., V 739-B27 to C.B.M.). We are grateful to F. Marr and C. Altmutter for excellent technical assistance and cell reconstruction, E. Kralli-Beller for manuscript editing, and the Scientific Service Units of IST Austria, especially T. Asenov and Miba machine shop, for maximally efficient support.
Author information
Authors and Affiliations
Contributions
D.V. and Y.O. performed the experiments and analyzed the data. P.J. and D.V. conceived the protocol and wrote the text. All authors analyzed data and jointly revised the protocol.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have the following competing financial interests: industrial collaboration with Leica Microsystems.
Additional information
Peer review information Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Vandael, D. et al. Neuron 107, 509–521.e7 (2020): https://doi.org/10.1016/j.neuron.2020.05.013
Supplementary information
Supplementary Video 1
MFB–CA3 pyramidal neuron paired recording under experimental conditions.
Rights and permissions
About this article
Cite this article
Vandael, D., Okamoto, Y., Borges-Merjane, C. et al. Subcellular patch-clamp techniques for single-bouton stimulation and simultaneous pre- and postsynaptic recording at cortical synapses. Nat Protoc 16, 2947–2967 (2021). https://doi.org/10.1038/s41596-021-00526-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00526-0
This article is cited by
-
Transsynaptic modulation of presynaptic short-term plasticity in hippocampal mossy fiber synapses
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.