Abstract
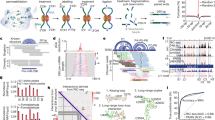
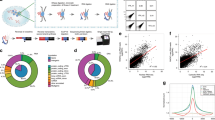
Emerging evidence has demonstrated that RNA-RNA interactions are vital in controlling diverse biological processes, including transcription, RNA splicing and protein translation. RNA in situ conformation sequencing (RIC-seq) is a technique for capturing protein-mediated RNA-RNA proximal interactions globally in living cells at single-base resolution. Cells are first treated with formaldehyde to fix all the protein-mediated RNA-RNA interactions in situ. After cell permeabilization and micrococcal nuclease digestion, the proximally interacting RNAs are 3′ end-labeled with pCp-biotin and subsequently ligated using T4 RNA ligase. The chimeric RNAs are then enriched and converted into libraries for paired-end sequencing. After deep sequencing, computational analysis yields interaction strength scores for every base on proximally interacting RNAs in the starting populations. The whole experimental procedure is designed to be completed within 6 d, followed by an additional 8 d for computational analysis. RIC-seq technology can unbiasedly detect intra- and intermolecular RNA-RNA interactions, thereby rendering it useful for reconstructing RNA higher-order structures and identifying direct noncoding RNA targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
RIC-seq data for HeLa cells (originally published in ref. 36) are available in the Gene Expression Omnibus under accession number GSE127188. An example dataset is deposited at https://drive.google.com/drive/folders/1HFjcE2LwbPFsmQy4h39wBZb2 cKCJIyB-?usp=sharing.
Code availability
The scripts for RIC-seq data analysis can be freely downloaded from GitHub at https://github.com/caochch/RICpipe.
References
Djebali, S. et al. Landscape of transcription in human cells. Nature 489, 101–108 (2012).
Chen, J. & Xue, Y. Emerging roles of non-coding RNAs in epigenetic regulation. Sci. China Life Sci. 59, 227–235 (2016).
Palazzo, A. F. & Lee, E. S. Non-coding RNA: what is functional and what is junk? Front. Genet. 6, 2 (2015).
Butcher, S. E. & Pyle, A. M. The molecular interactions that stabilize RNA tertiary structure: RNA motifs, patterns, and networks. Acc. Chem. Res. 44, 1302–1311 (2011).
Pyle, A. M. Looking at LncRNAs with the ribozyme toolkit. Mol. Cell 56, 13–17 (2014).
Guil, S. & Esteller, M. RNA-RNA interactions in gene regulation: the coding and noncoding players. Trends Biochem. Sci. 40, 248–256 (2015).
Steitz, J. RNA-RNA base-pairing: theme and variations. RNA 21, 476–477 (2015).
Wan, Y., et al. Understanding the transcriptome through RNA structure. Nat. Rev. Genet. 12, 641–655 (2011).
Aw, J. G. et al. In vivo mapping of eukaryotic RNA interactomes reveals principles of higher-order organization and regulation. Mol. Cell 62, 603–617 (2016).
Ding, Y. et al. In vivo genome-wide profiling of RNA secondary structure reveals novel regulatory features. Nature 505, 696–700 (2014).
Kertesz, M. et al. Genome-wide measurement of RNA secondary structure in yeast. Nature 467, 103–107 (2010).
Lu, Z. et al. RNA duplex map in living cells reveals higher-order transcriptome structure. Cell 165, 1267–1279 (2016).
Lucks, J. B. et al. Multiplexed RNA structure characterization with selective 2′-hydroxyl acylation analyzed by primer extension sequencing (SHAPE-Seq). Proc. Natl Acad. Sci. USA 108, 11063–11068 (2011).
Rouskin, S., et al. Genome-wide probing of RNA structure reveals active unfolding of mRNA structures in vivo. Nature 505, 701–705 (2014).
Sharma, E., Sterne-Weiler, T., O’Hanlon, D. & Blencowe, B. J. Global mapping of human RNA-RNA interactions. Mol. Cell 62, 618–626 (2016).
Smola, M. J., et al. Selective 2′-hydroxyl acylation analyzed by primer extension and mutational profiling (SHAPE-MaP) for direct, versatile and accurate RNA structure analysis. Nat. Protoc. 10, 1643–1669 (2015).
Spitale, R. C. et al. Structural imprints in vivo decode RNA regulatory mechanisms. Nature 519, 486–490 (2015).
Talkish, J., et al. Mod-seq: high-throughput sequencing for chemical probing of RNA structure. RNA 20, 713–720 (2014).
Underwood, J. G. et al. FragSeq: transcriptome-wide RNA structure probing using high-throughput sequencing. Nat. Methods 7, 995–1001 (2010).
Gerstberger, S., Hafner, M. & Tuschl, T. A census of human RNA-binding proteins. Nat. Rev. Genet. 15, 829–845 (2014).
Castello, A. et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012).
Castello, A. et al. Comprehensive identification of RNA-binding domains in human cells. Mol. Cell 63, 696–710 (2016).
Lee, F. C. Y. & Ule, J. Advances in CLIP technologies for studies of protein-RNA interactions. Mol. Cell 69, 354–369 (2018).
Xue, Y. et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell 152, 82–96 (2013).
Xue, Y. et al. Genome-wide analysis of PTB-RNA interactions reveals a strategy used by the general splicing repressor to modulate exon inclusion or skipping. Mol. Cell 36, 996–1006 (2009).
Helwak, A., Kudla, G., Dudnakova, T. & Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153, 654–665 (2013).
Shen, E. Z. et al. Identification of piRNA binding sites reveals the Argonaute regulatory landscape of the C. elegans germline. Cell 172, 937–951.e18 (2018).
Kudla, G. et al. Cross-linking, ligation, and sequencing of hybrids reveals RNA-RNA interactions in yeast. Proc. Natl Acad. Sci. USA 108, 10010–10015 (2011).
Sugimoto, Y. et al. hiCLIP reveals the in vivo atlas of mRNA secondary structures recognized by Staufen 1. Nature 519, 491–494 (2015).
Song, Y. et al. irCLASH reveals RNA substrates recognized by human ADARs. Nat. Struct. Mol. Biol. 27, 351–362 (2020).
Ramani, V., Qiu, R. & Shendure, J. High-throughput determination of RNA structure by proximity ligation. Nat. Biotechnol. 33, 980–984 (2015).
Nguyen, T. C. et al. Mapping RNA-RNA interactome and RNA structure in vivo by MARIO. Nat. Commun. 7, 12023 (2016).
Nagano, T. et al. Comparison of Hi-C results using in-solution versus in-nucleus ligation. Genome Biol. 16, 175 (2015).
Quinodoz, S. A. et al. Higher-order inter-chromosomal hubs shape 3D genome organization in the nucleus. Cell 174, 744–757.e24 (2018).
Li, P. et al. Integrative analysis of Zika virus genome RNA structure reveals critical determinants of viral infectivity. Cell Host Microbe 24, 875–886.e5 (2018).
Cai, Z. et al. RIC-seq for global in situ profiling of RNA-RNA spatial interactions. Nature 582, 432–437 (2020).
Preker, P. et al. RNA exosome depletion reveals transcription upstream of active human promoters. Science 322, 1851–1854 (2008).
Core, L. J. et al. Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nat. Genet. 46, 1311–1320 (2014).
Metkar, M. et al. Higher-order organization principles of pre-translational mRNPs. Mol. Cell 72, 715–726.e3 (2018).
Parkhomchuk, D. et al. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 37, e123 (2009).
Ule, J. et al. CLIP identifies Nova-regulated RNA networks in the brain. Science 302, 1212–1215 (2003).
Ingolia, N. T., et al. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 7, 1534–1550 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Adiconis, X. et al. Comparative analysis of RNA sequencing methods for degraded or low-input samples. Nat. Methods 10, 623–629 (2013).
Morf, J. et al. RNA proximity sequencing reveals the spatial organization of the transcriptome in the nucleus. Nat. Biotechnol. 37, 793–802 (2019).
Anger, A. M. et al. Structures of the human and Drosophila 80S ribosome. Nature 497, 80–85 (2013).
Thorvaldsdottir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 (2013).
Acknowledgements
We thank Jing Hu for the critical review of this manuscript. This work was supported by the Ministry of Science and Technology of China (2017YFA0504400), the National Natural Science Foundation of China (32025008, 91740201, 91940306, 31522015 and 81921003), the Strategic Priority Program of CAS (XDB37000000; to Y.X.), the Beijing Municipal Natural Science Foundation (5182024) and the National Natural Science Foundation of China (31900465; to C.C).
Author information
Authors and Affiliations
Contributions
Z.C. and Y.X. designed, implemented and optimized the original RIC-seq experimental protocol with the help of R.Y. and R.S. C.C. designed the data-processing pipeline. N.H. and H.Z. tested the computational pipeline. Y.X., C.C. and Z.C. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.C., Z.C. and Y.X. have filed a patent application for RIC-seq technology with the application number 201910384194.2.
Additional information
Peer review information Nature Protocols thanks Rory Johnson, John Rinn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Cai, Z. et al. Nature 582, 432–437 (2020): https://doi.org/10.1038/s41586-020-2249-1
Rights and permissions
About this article
Cite this article
Cao, C., Cai, Z., Ye, R. et al. Global in situ profiling of RNA-RNA spatial interactions with RIC-seq. Nat Protoc 16, 2916–2946 (2021). https://doi.org/10.1038/s41596-021-00524-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00524-2
This article is cited by
-
Complementary Alu sequences mediate enhancer–promoter selectivity
Nature (2023)
-
Recent advances in RNA structurome
Science China Life Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.