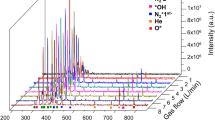
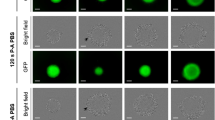
Abstract
Cold atmospheric plasma (CAP) is a potential anticancer therapy. CAP has cytotoxic effects when applied either directly to cancer cell cultures or indirectly through plasma-conditioned liquids. This protocol describes how to treat adherent cultures of human cancer cell lines with CAP or plasma-conditioned medium and determine cell viability following treatment. The protocol also includes details on how to quantify the reactive oxygen and nitrogen species present in medium following CAP treatment, using chemical probes using UV-visible or fluorescence spectroscopy. CAP treatment takes ~30 min, and 3 h are required to complete quantification of reactive oxygen and nitrogen species. By providing a standardized protocol for evaluation of the effects of CAP and plasma-conditioned medium, we hope to facilitate the comparison and interpretation of results seen across different laboratories.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
Source data are available for Figs. 8–10 at https://upcommons.upc.edu/handle/2117/330395.
References
Lis, K. A. et al. Inactivation of multidrug-resistant pathogens and Yersinia enterocolitica with cold atmospheric-pressure plasma on stainless-steel surfaces. Int. J. Antimicrob. Agents 52, 811–818 (2018).
Brun, P. et al. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 125, 398–408 (2018).
Yang, Y. et al. A novel cold atmospheric pressure air plasma jet for peri-implantitis treatment: An in vitro study. Dent. Mater. J. 37, 157–166 (2018).
Klämpfl, T. G. et al. Decontamination of nosocomial bacteria including clostridium difficile spores on dry inanimate surface by cold atmospheric plasma. Plasma Process. Polym. 11, 974–984 (2014).
Daeschlein, G. et al. Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. J. Dtsch. Dermatol. Ges. 13, 143–150 (2015).
Arndt, S., Unger, P., Berneburg, M., Bosserhoff, A. K. & Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 89, 181–190 (2018).
Duchesne, C., Banzet, S., Lataillade, J. J., Rousseau, A. & Frescaline, N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J. Pathol. 249, 368–380 (2019).
Nomura, Y. et al. Investigation of blood coagulation effect of nonthermal multigas plasma jet in vitro and in vivo. J. Surg. Res. 219, 302–309 (2017).
Schuster, M. et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Craniomaxillofac. Surg. 44, 1445–1452 (2016).
Isbary, G. et al. Cold atmospheric plasma for local infection control and subsequent pain reduction in a patient with chronic post-operative ear infection. New Microbes New Infect. 1, 41–43 (2013).
Heinlin, J. et al. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. 25, 1–11 (2011).
Dubuc, A. et al. Use of cold-atmospheric plasma in oncology: a concise systematic review. Ther. Adv. Med. Oncol. 10, 1758835918786475 (2018).
Bauer, G., Sersenova, D., Graves, D. B. & Machala, Z. Cold atmospheric plasma and plasma-activated medium trigger RONS-based tumor cell apoptosis. Sci. Rep. 9, 14210 (2019).
Tornin, J. et al. Pyruvate plays a main role in the antitumoral selectivity of cold atmospheric plasma in osteosarcoma. Sci. Rep. 9, 10681 (2019).
Cheng, X. et al. The effect of tuning cold plasma composition on glioblastoma cell viability. PLoS ONE 9, e98652 (2014).
Canal, C. et al. Plasma-induced selectivity in bone cancer cells death. Free Radic. Biol. Med. 110, 72–80 (2017).
Labay, C., Hamouda, I., Tampieri, F., Ginebra, M. P. & Canal, C. Production of reactive species in alginate hydrogels for cold atmospheric plasma-based therapies. Sci. Rep. 9, 16160 (2019).
Mateu-Sanz, M. et al. Cold plasma-treated Ringer’s saline: a weapon to target osteosarcoma. Cancers (Basel) https://doi.org/10.3390/cancers12010227 (2020).
Khlyustova, A., Labay, C., Machala, Z., Ginebra, M.-P. & Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: a brief review. Front. Chem. Sci. Eng. 13, 238–252 (2019).
Tanaka, H. et al. Plasma-activated medium selectively kills glioblastoma brain tumor cells by down-regulating a survival signaling molecule, AKT kinase. Plasma Med. https://doi.org/10.1615/PlasmaMed.2012006275 (2011).
Tanaka, H. et al. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 6, 36282 (2016).
Girard, P. M. et al. Synergistic effect of H2O2 and NO2 in cell death induced by cold atmospheric He plasma. Sci. Rep. 6, 29098 (2016).
Schmidt, A. et al. Cold physical plasma modulates p53 and mitogen-activated protein kinase signaling in keratinocytes. Oxid. Med. Cell Longev. 2019, 7017363 (2019).
Yan, D. et al. The specific vulnerabilities of cancer cells to the cold atmospheric plasma-stimulated solutions. Sci. Rep. 7, 4479 (2017).
Tornín, J., Villasante, A., Solé-Martí, X., Ginebra, M.-P. & Canal, C. Osteosarcoma tissue-engineered model challenges oxidative stress therapy revealing promoted cancer stem cell properties. Free Radic. Biol. Med. https://doi.org/10.1016/j.freeradbiomed.2020.12.437 (2021).
Bauer, G. Targeting protective catalase of tumor cells with cold atmospheric plasma- activated medium (PAM). Anti-Cancer Agents in Medicinal Chemistry 18, 784–804 (2018).
Yan, D. et al. The strong cell-based hydrogen peroxide generation triggered by cold atmospheric plasma. Sci. Rep. 7, 10831 (2017).
Guevara, I. et al. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta 274, 177–188 (1998).
Kim, S. J. & Chung, T. H. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 6, 20332 (2016).
Akter, M., Jangra, A., Choi, S. A., Choi, E. H. & Han, I. Non-thermal atmospheric pressure bio-compatible plasma stimulates apoptosis via p38/MAPK mechanism in U87 malignant glioblastoma. Cancers (Basel) https://doi.org/10.3390/cancers12010245 (2020).
Bundscherer, L. et al. Impact of non-thermal plasma treatment on MAPK signaling pathways of human immune cell lines. Immunobiology 218, 1248–1255 (2013).
Kang, S. U. et al. Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 5, e1056 (2014).
Kaushik, N. et al. Responses of solid tumor cells in DMEM to reactive oxygen species generated by non-thermal plasma and chemically induced ROS systems. Sci. Rep. 5, 8587 (2015).
Xiang, L., Xu, X., Zhang, S., Cai, D. & Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Rad. Biol. Med. 124, 205–213 (2018).
Schmidt, A. et al. Role of ambient gas composition on cold physical plasma-elicited cell signaling in keratinocytes. Biophys. J. 112, 2397–2407 (2017).
Bekeschus, S. et al. Risk assessment of kINPen plasma treatment of four human pancreatic cancer cell lines with respect to metastasis. Cancers (Basel) https://doi.org/10.3390/cancers11091237 (2019).
Bekeschus, S. et al. Neutrophil extracellular trap formation is elicited in response to cold physical plasma. J. Leukoc. Biol. 100, 791–799 (2016).
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 714793). Authors belong to the SGR2017 1165. M.P.G. and C.C. acknowledge the Generalitat de Catalunya for the ICREA Academia Award. Authors acknowledge the technical support of M. Sánchez.
Author information
Authors and Affiliations
Contributions
J.T. and C.L. contributed to the acquisition, analysis and interpretation of data, optimization of the protocol and writing of the work. F.T. contributed to acquisition and analysis of data, and to revising it. M.P.G. contributed to writing, discussing and revising the work. C.C. conceived the work, contributed to writing and substantively revising it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Sander Bekeschus, Hiromasa Tanaka and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Tornin, J. et al. Sci. Rep. 9, 10681 (2019): https://doi.org/10.1038/s41598-019-47128-1
Mateu-Sanz, M. et al. Cancers 12, 227 (2020): https://doi.org/10.3390/cancers12010227
Tornin, J. et al. Free Rad. J Biol. Med. 164, 107 (2021): https://doi.org/10.1016/j.freeradbiomed.2020.12.437
Supplementary information
Supplementary Information
Supplementary Table 1 and Supplementary Methods 1 and 2.
Rights and permissions
About this article
Cite this article
Tornin, J., Labay, C., Tampieri, F. et al. Evaluation of the effects of cold atmospheric plasma and plasma-treated liquids in cancer cell cultures. Nat Protoc 16, 2826–2850 (2021). https://doi.org/10.1038/s41596-021-00521-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00521-5
This article is cited by
-
Cold atmospheric plasma stabilizes mismatch repair for effective, uniform treatment of diverse colorectal cancer cell types
Scientific Reports (2024)
-
Cold atmospheric plasma differentially affects cell renewal and differentiation of stem cells and APC-deficient-derived tumor cells in intestinal organoids
Cell Death Discovery (2022)
-
Efficacy of plasma activated saline in a co-culture infection control model
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.