Abstract
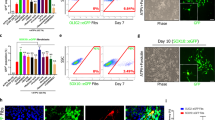
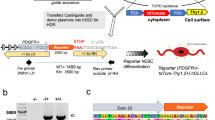
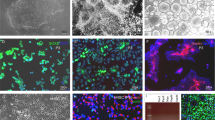
Oligodendrocytes (OLs) are responsible for myelin production and metabolic support of neurons. Defects in OLs are crucial in several neurodegenerative diseases including multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS). This protocol describes a method to generate oligodendrocyte precursor cells (OPCs) from human pluripotent stem cells (hPSCs) in only ~20 d, which can subsequently myelinate neurons, both in vitro and in vivo. To date, OPCs have been derived from eight different hPSC lines including those derived from patients with spontaneous and familial forms of MS and ALS, respectively. hPSCs, fated for 8 d toward neural progenitors, are transduced with an inducible lentiviral vector encoding for SOX10. The addition of doxycycline for 10 d results in >60% of cells being O4-expressing OPCs, of which 20% co-express the mature OL marker myelin basic protein (MBP). The protocol also describes an alternative for viral transduction, by incorporating an inducible SOX10 in the safe harbor locus AAVS1, yielding ~100% pure OPCs. O4+ OPCs can be purified and either cryopreserved or used for functional studies. As an example of the type of functional study for which the derived cells could be used, O4+ cells can be co-cultured with maturing hPSC-derived neurons in 96/384-well-format plates, allowing the screening of pro-myelinating compounds.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Plasmids used and derived during this procedure are available through Addgene: https://www.addgene.org/browse/article/28196370/ and https://www.addgene.org/browse/article/28195986/. The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request or available as source data files associated with individual figures. Source data are provided with this paper.
References
Franklin, R. J. M., Ffrench-Constant, C., Edgar, J. M. & Smith, K. J. Neuroprotection and repair in multiple sclerosis. Nat. Rev. Neurol. 8, 624–634 (2012).
Li, L. et al. GFAP mutations in astrocytes impair oligodendrocyte progenitor proliferation and myelination in an hiPSC model of Alexander disease. Cell Stem Cell 23, 239–251.e6 (2018).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Osipovitch, M. et al. Human ESC-derived chimeric mouse models of Huntington’s disease reveal cell-intrinsic defects in glial progenitor cell differentiation. Cell Stem Cell 24, 107–122.e7 (2019).
Windrem, M. S. et al. Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell 21, 195–208.e6 (2017).
de Vrij, F. M. et al. Candidate CSPG4 mutations and induced pluripotent stem cell modeling implicate oligodendrocyte progenitor cell dysfunction in familial schizophrenia. Mol. Psychiatry 24, 757–771 (2019).
Garcia-Leon, J. A., Vitorica, J. & Gutierrez, A. Use of human pluripotent stem cell-derived cells for neurodegenerative disease modeling and drug screening platform. Future Med. Chem. 11, 1305–1322 (2019).
García-León, J. A. et al. SOX10 single transcription factor-based fast and efficient generation of oligodendrocytes from human pluripotent stem cells. Stem Cell Reports 10, 655–672 (2018).
Goldman, S. A. & Kuypers, N. J. How to make an oligodendrocyte. Development 142, 3983–3995 (2015).
Liu, Y., Jiang, P. & Deng, W. OLIG gene targeting in human pluripotent stem cells for motor neuron and oligodendrocyte differentiation. Nat. Protoc. 6, 640–655 (2011).
Nistor, G. I., Totoiu, M. O., Haque, N., Carpenter, M. K. & Keirstead, H. S. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 49, 385–396 (2005).
Kang, S.-M. et al. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells 25, 419–424 (2007).
Izrael, M. et al. Human oligodendrocytes derived from embryonic stem cells: effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol. Cell. Neurosci. 34, 310–323 (2007).
Hu, B.-Y., Du, Z.-W. & Zhang, S.-C. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat. Protoc. 4, 1614–1622 (2009).
Sundberg, M., Skottman, H., Suuronen, R. & Narkilahti, S. Production and isolation of NG2+ oligodendrocyte precursors from human embryonic stem cells in defined serum-free medium. Stem Cell Res. 5, 91–103 (2010).
Stacpoole, S. R. L. et al. High yields of oligodendrocyte lineage cells from human embryonic stem cells at physiological oxygen tensions for evaluation of translational biology. Stem Cell Reports 1, 437–450 (2013).
Jang, J. et al. Induced pluripotent stem cell models from X-linked adrenoleukodystrophy patients. Ann. Neurol. 70, 402–409 (2011).
Pouya, A., Satarian, L., Kiani, S., Javan, M. & Baharvand, H. Human induced pluripotent stem cells differentiation into oligodendrocyte progenitors and transplantation in a rat model of optic chiasm demyelination. PLoS One 6, e27925 (2011).
Wang, S. et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12, 252–264 (2013).
Douvaras, P. et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Reports 3, 250–259 (2014).
Douvaras, P. & Fossati, V. Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nat. Protoc. 10, 1143–1154 (2015).
Zhang, Y. et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013).
Wang, J. et al. Transcription factor induction of human oligodendrocyte progenitor fate and differentiation. Proc. Natl Acad. Sci. USA 111, E2885–E2894 (2014).
Chanoumidou, K., Mozafari, S., Baron‐Van Evercooren, A. & Kuhlmann, T. Stem cell derived oligodendrocytes to study myelin diseases. Glia 68, 705–720 (2019).
Pawlowski, M. et al. Inducible and deterministic forward programming of human pluripotent stem cells into neurons, skeletal myocytes, and oligodendrocytes. Stem Cell Reports 8, 803–812 (2017).
Ehrlich, M. et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc. Natl Acad. Sci. USA 114, E2243–E2252 (2017).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Patani, R. et al. Retinoid-independent motor neurogenesis from human embryonic stem cells reveals a medial columnar ground state. Nat. Commun. 2, 214 (2011).
Smith, J. R. et al. Robust, persistent transgene expression in human embryonic stem cells is achieved with AAVS1-targeted integration. Stem Cells 26, 496–504 (2008).
Ordovás, L. et al. Efficient recombinase-mediated cassette exchange in hPSCs to study the hepatocyte lineage reveals AAVS1 locus-mediated transgene inhibition. Stem Cell Reports 5, 918–931 (2015).
Shi, Y., Kirwan, P. & Livesey, F. J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc. 7, 1836–1846 (2012).
Raitano, S. et al. Restoration of progranulin expression rescues cortical neuron generation in an induced pluripotent stem cell model of frontotemporal dementia. Stem Cell Reports 4, 16–24 (2015).
Fumagalli, L. et al. C9orf72-derived arginine-containing dipeptide repeats associate with axonal transport machinery and impede microtubule-based motility. Preprint at https://www.biorxiv.org/content/10.1101/835082v1 (2019).
García-León, J. A. et al. Generation of a human induced pluripotent stem cell–based model for tauopathies combining three microtubule-associated protein tau mutations which displays several phenotypes linked to neurodegeneration. Alzheimer’s Dement. 14, 1261–1280 (2018).
Acknowledgements
We acknowledge technical colleagues at SCIL (KU Leuven) for their technical support. J.A.G.-L has been supported by a Fellowship from Alfonso Martín Escudero Foundation (Madrid, Spain), by a contract of doctor reincorporation plan from the I Plan Propio of the University of Malaga (Spain) and by CIBERNED. The work was supported by an IWT-iPSCAF grant (no. 150031) and KUL-PF Stem Cells (no. PFO3) to C.M.V, by Instituto de Salud Carlos III (ISCiii) of Spain, cofinanced by FEDER funds from the European Union (grant PI18/01557 to A.G.), by Junta de Andalucia grants UMA18-FEDERJA-211 and PY18-RT-2233 (to A.G.), co-financed by Programa Operativo FEDER 2014-2020, by Consejeria de Salud of Junta de Andalucia (grant PI-0276-2018 to J.A.G.-L.) and by CIBERNED (grant CB06/05/1116 to A.G.). In vivo studies were supported by the Progressive MS Alliance (PMSA, collaborative research network PA-1604-08492 (BRAVEinMS)), INSERM and ICM grants to A.B.V.-E. and Junta de Andalucía and the European Commission under the Seventh Framework Program of the European Union (agreement no. 291730, contract TAHUB-II-107) to B.G-D. The authors thank the ICM animal, genomic, culture and cellular imaging core facilities.
Author information
Authors and Affiliations
Contributions
J.A.G.-L. and C.M.V. conceived the study and generated the protocol. J.A.G.-L., K.E., L.C.-P., K.N. and R.M.d.C. performed the experiments and analysis of the data. B.G.D. performed the experiments of the shiverer mouse. J.C.D. assisted with electron microscopy. A.B.V.-E. provided scientific support and guidance on the in vivo experiments. A.G. provided scientific guidance and support. J.A.G.-L. and C.M.V. wrote the manuscript with input from B.G.D. and A.B.V.-E. C.M.V. provided scientific guidance and support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
García-León, J. A. et al. Stem Cell Reports 10, 655–672 (2018): https://doi.org/10.1016/j.stemcr.2017.12.014
Key data used in this protocol
García-León, J. A. et al. Stem Cell Reports 10, 655–672 (2018): https://doi.org/10.1016/j.stemcr.2017.12.014
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Supplementary Methods.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
García-León, J.A., García-Díaz, B., Eggermont, K. et al. Generation of oligodendrocytes and establishment of an all-human myelinating platform from human pluripotent stem cells. Nat Protoc 15, 3716–3744 (2020). https://doi.org/10.1038/s41596-020-0395-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0395-4
This article is cited by
-
Gliomas: a reflection of temporal gliogenic principles
Communications Biology (2024)
-
Disruption of MAM integrity in mutant FUS oligodendroglial progenitors from hiPSCs
Acta Neuropathologica (2024)
-
Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke
Cell Death Discovery (2023)
-
From methylation to myelination: epigenomic and transcriptomic profiling of chronic inactive demyelinated multiple sclerosis lesions
Acta Neuropathologica (2023)
-
Rapid differentiation of hiPSCs into functional oligodendrocytes using an OLIG2 synthetic modified messenger RNA
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.