Abstract
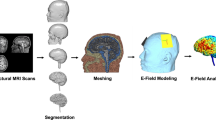
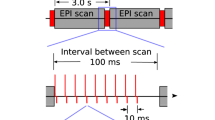
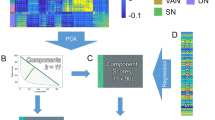
Transcranial magnetic stimulation (TMS) is a noninvasive method to stimulate the cerebral cortex that has applications in psychiatry, such as in the treatment of depression and anxiety. Although many TMS targeting methods that use figure-8 coils exist, many do not account for individual differences in anatomy or are not generalizable across target sites. This protocol combines functional magnetic resonance imaging (fMRI) and iterative electric-field (E-field) modeling in a generalized approach to subject-specific TMS targeting that is capable of optimizing the stimulation site and TMS coil orientation. To apply this protocol, the user should (i) operationally define a region of interest (ROI), (ii) generate the head model from the structural MRI data, (iii) preprocess the functional MRI data, (iv) identify the single-subject stimulation site within the ROI, and (iv) conduct E-field modeling to identify the optimal coil orientation. In comparison with standard targeting methods, this approach demonstrates (i) reduced variability in the stimulation site across subjects, (ii) reduced scalp-to-cortical-target distance, and (iii) reduced variability in optimal coil orientation. Execution of this protocol requires intermediate-level skills in structural and functional MRI processing. This protocol takes ~24 h to complete and demonstrates how constrained fMRI targeting combined with iterative E-field modeling can be used as a general method to optimize both the TMS coil site and its orientation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Example data have been uploaded as Supplementary Data.
Code availability
The code has been uploaded to GitHub (https://github.com/balders2/tms_targeting).
References
Laakso, I., Murakami, T., Hirata, A. & Ugawa, Y. Where and what TMS activates: experiments and modeling. Brain Stimul. 11, 166–174 (2017).
Terao, Y. & Ugawa, Y. Basic mechanisms of TMS. J. Clin. Neurophysiol. 19, 322–343 (2002).
Thielscher, A. & Kammer, T. Electric field properties of two commercial figure-8 coils in TMS: calculation of focality and efficiency. Clin. Neurophysiol. 115, 1697–1708 (2004).
Roth, Y., Amir, A., Levkovitz, Y. & Zangen, A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J. Clin. Neurophysiol. 24, 31–38 (2007).
Deng, Z.-D., Lisanby, S. H. & Peterchev, A. V. Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 6, 1–13 (2013).
Rogasch, N. C., Daskalakis, Z. J. & Fitzgerald, P. B. Cortical inhibition of distinct mechanisms in the dorsolateral prefrontal cortex is related to working memory performance: a TMS-EEG study. Cortex 64, 68–77 (2015).
Bona, S., Herbert, A., Toneatto, C., Silvanto, J. & Cattaneo, Z. The causal role of the lateral occipital complex in visual mirror symmetry detection and grouping: an fMRI-guided TMS study. Cortex 51, 46–55 (2014).
Luber, B. M. et al. Remediation of sleep-deprivation-induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb. Cortex 18, 2077–2085 (2008).
Luber, B. M. et al. Extended remediation of sleep deprived-induced working memory deficits using fMRI-guided transcranial magnetic stimulation. Sleep 36, 857–871 (2013).
Luber, B. M. et al. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency- and time-dependent effects. Brain Res. 1128, 120–129 (2007).
Weiss, C. et al. Mapping the hand, foot and face representations in the primary motor cortex – retest reliability of neuronavigated TMS versus functional MRI. Neuroimage 66, 531–542 (2013).
Sarfeld, A. S. et al. Convergence of human brain mapping tools: neuronavigated TMS parameters and fMRI activity in the hand motor area. Hum. Brain Mapp. 33, 1107–1123 (2012).
Ahdab, R., Ayache, S. S., Brugières, P., Goujon, C. & Lefaucheur, J.-P. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol. Clin. Neurophysiol. 40, 27–36 (2010).
Nauczyciel, C. et al. Assessment of standard coil positioning in transcranial magnetic stimulation in depression. Psychiatry Res. 186, 232–238 (2011).
O’Reardon, J. P. et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry 62, 1208–1216 (2007).
Horvath, J. C., Mathews, J., Demitrack, M. A & Pascual-Leone, A. The NeuroStar TMS device: conducting the FDA approved protocol for treatment of depression. J. Vis. Exp. 2010, e2345 (2010).
Davis, S. W., Luber, B., Murphy, D. L. K., Lisanby, S. H. & Cabeza, R. Frequency-specific neuromodulation of local and distant connectivity in aging and episodic memory function. Hum. Brain Mapp. 38, 5987–6004 (2017).
Luber, B. M. et al. Reprint of “Using neuroimaging to individualize TMS treatment for depression: toward a new paradigm for imaging-guided intervention”. Neuroimage 151, 65–71 (2017).
Sack, A. T. et al. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J. Cogn. Neurosci. 21, 207–221 (2009).
Balderston, N. L. et al. Anxiety patients show reduced working memory related dlpfc activation during safety and threat. Depress Anxiety 12, 1–12 (2016).
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Glover, G. H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 22, 133–139 (2011).
Poldrack, R. A. Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 10, 59–63 (2006).
Mather, M., Cacioppo, J. T. & Kanwisher, N. How fMRI can inform cognitive theories. Perspect. Psychol. Sci. 8, 108–113 (2013).
Wilson, C. R. E., Gaffan, D., Browning, P. G. F. & Baxter, M. G. Functional localization within the prefrontal cortex: missing the forest for the trees? Trends Neurosci. 33, 533–540 (2010).
Juch, H., Zimine, I., Seghier, M. L., Lazeyras, F. & Fasel, J. H. D. Anatomical variability of the lateral frontal lobe surface: implication for intersubject variability in language neuroimaging. Neuroimage 24, 504–514 (2005).
Smith, S. M. et al. Variability in fMRI: a re-examination of inter-session differences. Hum. Brain Mapp. 24, 248–257 (2005).
Bijsterbosch, J. D., Barker, A. T., Lee, K. H. & Woodruff, P. W. R. Where does transcranial magnetic stimulation (TMS) stimulate? Modelling of induced field maps for some common cortical and cerebellar targets. Med. Biol. Eng. Comput. 50, 671–681 (2012).
Janssen, A. M., Oostendorp, T. F. & Stegeman, D. F. The effect of local anatomy on the electric field induced by TMS: evaluation at 14 different target sites. Med. Biol. Eng. Comput. 52, 873–883 (2014).
Krieg, T. D., Salinas, F. S., Narayana, S., Fox, P. T. & Mogul, D. J. Computational and experimental analysis of TMS-induced electric field vectors critical to neuronal activation. J. Neural Eng. 12, 046014 (2015).
Opitz, A., Paulus, W., Will, S., Antunes, A. & Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage 109, 140–150 (2015).
De Geeter, N., Crevecoeur, G., Leemans, A. & Dupr, L. Effective electric fields along realistic DTI-based neural trajectories for modelling the stimulation mechanisms of TMS. Phys. Med. Biol. 60, 453–471 (2015).
Salinas, F. S., Lancaster, J. L. & Fox, P. T. 3D modeling of the total electric field induced by transcranial magnetic stimulation using the boundary element method. Phys. Med. Biol. 54, 3631–3647 (2009).
Salinas, F. S., Lancaster, J. L. & Fox, P. T. Detailed 3D models of the induced electric field of transcranial magnetic stimulation coils. Phys. Med. Biol. 52, 2879–2892 (2007).
Seo, H. & Jun, S. C. Multi-scale computational models for electrical brain stimulation. Front. Hum. Neurosci. 1–14 (2017).
Tachas, N. J. & Samaras, T. The effect of head and coil modeling for the calculation of induced electric field during transcranial magnetic stimulation. Int. J. Psychophysiol. 93, 167–171 (2014).
Thielscher, A., Antunes, A. & Saturnino, G. B. Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 222–225 (Milan, 2015). https://doi.org/10.1109/EMBC.2015.7318340
Balderston, N. L. et al. Mechanistic link between right prefrontal cortical activity and anxious arousal revealed using transcranial magnetic stimulation in healthy subjects. Neuropsychopharmacology 45, 694–702 (2020).
Balderston, N. L. et al. Low-frequency parietal repetitive transcranial magnetic stimulation reduces fear and anxiety. Transl. Psychiatry 10, 68 (2020).
Balderston, N. L. et al. Threat of shock increases excitability and connectivity of the intraparietal sulcus. Elife 6, e23608 (2017).
Weise, K., Numssen, O., Thielscher, A., Hartwigsen, G. & Knösche, T. R. A novel approach to localize cortical TMS effects. Neuroimage 209, 116486 (2020).
Aberra, A. S., Wang, B., Grill, W. M. & Peterchev, A. V. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 13, 175–189 (2020).
Sternberg, S. High-speed scanning in human memory. Science 153, 652–654 (1966).
Balderston, N. L., Hsiung, A., Liu, J., Ernst, M. & Grillon, C. Reducing state anxiety using working memory maintenance. J. Vis. Exp. 2017, 55727 (2017).
Balderston, N. L. et al. Working memory maintenance is sufficient to reduce state anxiety. Psychophysiology 53, 1660–1668 (2016).
Maxwell, J. C. A dynamical theory of the electromagnetic field. Philos. Trans. R. Soc. Lond. 155, 459–512 (1865).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Yarkoni, T., Poldrack, R., Nichols, T., Van Essen, D. & Wager, T. NeuroSynth: a new platform for large-scale automated synthesis of human functional neuroimaging data. Frontiers in Neuroinformatics Conference Abstract: 4th INCF Congress of Neuroinformatics (2011). https://doi.org/10.3389/conf.fninf.2011.08.00058
Altamura, M. et al. Dissociating the effects of Sternberg working memory demands in prefrontal cortex. Psychiatry Res. 154, 103–114 (2007).
Barbey, A. K., Koenigs, M. & Grafman, J. Dorsolateral prefrontal contributions to human working memory. Cortex 49, 1195–1205 (2013).
Acknowledgements
This study used the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, MD (https://hpc.nih.gov/). This project was supported in part by a 2018 NARSAD Young Investigator Grant from the Brain & Behavior Foundation (N.L.B.). Financial support for this study was provided by the Intramural Research Program of the National Institute of Mental Health (ZIAMH002798; ClinicalTrial.gov Identifier: NCT03027414: Protocol ID 17-M-0042). The authors all work at the National Institutes of Health. The views expressed here are the authors’ own and do not necessarily reflect the views of the NIH, DHHS, or the US federal government.
Author information
Authors and Affiliations
Contributions
The study was designed by N.L.B., C.G., M.E., B.L., and S.H.L. The protocol was designed by N.L.B., Z.-D.D., T.R., B.L., and S.H.L. The data were collected by N.L.B., C.R., and E.M.B., and were analyzed by N.L.B. The manuscript was prepared by N.L.B., B.L., S.H.L., M.E., and C.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Thomas Knoesche and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Balderston N. et al. Transl. Psychiatry 10, 68 (2020): https://doi.org/10.1038/s41398-020-0751-8
Balderston N. et al. Neuropsychopharmacology 45, 694–702 (2020): https://doi.org/10.1038/s41386-019-0583-5
Davis S. W., Luber, B., Murphy, D. L. K., Lisanby, S. H. & Cabeza, R. Hum. Brain Mapp. 38, 5987–6004 (2017): https://doi.org/10.1002/hbm.23803
Supplementary information
Supplementary Data
Example data to be used with the protocol.
Rights and permissions
About this article
Cite this article
Balderston, N.L., Roberts, C., Beydler, E.M. et al. A generalized workflow for conducting electric field–optimized, fMRI-guided, transcranial magnetic stimulation. Nat Protoc 15, 3595–3614 (2020). https://doi.org/10.1038/s41596-020-0387-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0387-4
This article is cited by
-
Accelerated TMS - moving quickly into the future of depression treatment
Neuropsychopharmacology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.