Abstract
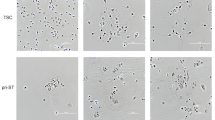
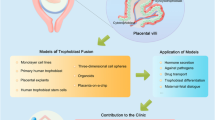
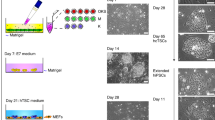
The human placenta is essential for successful reproduction. There is great variation in the anatomy and development of the placenta in different species, meaning that animal models provide limited information about human placental development and function. Until recently, it has been impossible to isolate trophoblast cells from the human placenta that proliferate in vitro. This has limited our ability to understand pregnancy disorders. Generating an in vitro model that recapitulates the unique features of the human placenta has been challenging. The first in vitro model system of human trophoblast that could be cultured long term and differentiated to syncytiotrophoblast (SCT) and extravillous trophoblast (EVT) was a two-dimensional (2D) culture system of human trophoblast stem cells. Here, we describe a protocol to isolate trophoblast from first-trimester human placentas that can be grown long term in a three-dimensional (3D) organoid culture system. Trophoblast organoids can be established within 2−3 weeks, passaged every 7–10 d, and cultured for over a year. The structural organization of these human trophoblast organoids closely resembles the villous placenta with a layer of cytotrophoblast (VCT) that differentiates into superimposed SCT. Altering the composition of the medium leads to differentiation of the trophoblast organoids into HLA-G+ EVT cells which rapidly migrate and invade through the Matrigel droplet in which they are cultured. Our previous research confirmed that there is similarity between the trophoblast organoids and in vivo placentas in their transcriptomes and ability to produce placental hormones. This organoid culture system provides an experimental model to investigate human placental development and function as well as interactions of trophoblast cells with the local and systemic maternal environment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Smith, G. C. S. First-trimester determination of complications of late pregnancy. JAMA 303, 561–562 (2010).
Hamilton, W. J. & Boyd, J. D. Development of the human placenta in the first three months of gestation. J. Anat. 94, 297–328 (1960).
Lee, C. Q. E. et al. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Rep. 6, 257–272 (2016).
Turco, M. Y. & Moffett, A. Development of the human placenta. Development 146, dev163428 (2019).
Horii, M., Touma, O., Bui, T. & Parast, M. Modeling human trophoblast, the placental epithelium at the maternal fetal interface. Reproduction 160, R1–R11 (2020).
Tanaka, S., Kunath, T., Hadjantonakis, A.-K., Nagy, A. & Rossant, J. Promotion of trophoblast stem cell proliferation by FGF4. Science 282, 2072 LP–2072075 (1998).
Okae, H. et al. Derivation of human trophoblast stem cells. Cell Stem Cell 22, 50–63.e6 (2018).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 564, 263–267 (2018).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Kunath, T. et al. Developmental differences in the expression of FGF receptors between human and mouse embryos. Placenta 35, 1079–1088 (2014).
Paiva, P. et al. Human chorionic gonadotrophin regulates FGF2 and other cytokines produced by human endometrial epithelial cells, providing a mechanism for enhancing endometrial receptivity. Hum. Reprod. 26, 1153–1162 (2011).
Hempstock, J., Cindrova-Davies, T., Jauniaux, E. & Burton, G. J. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod. Biol. Endocrinol. 2, 58 (2004).
Mühlhauser, J. et al. Differentiation and proliferation patterns in human trophoblast revealed by c-erbB-2 oncogene product and EGF-R. J. Histochem. Cytochem. 41, 165–173 (1993).
Sonderegger, S., Husslein, H., Leisser, C. & Knöfler, M. Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta 28, S97–S102 (2007).
Haider, S., Kunihs, V., Fiala, C., Pollheimer, J. & Knöfler, M. Expression pattern and phosphorylation status of Smad2/3 in different subtypes of human first trimester trophoblast. Placenta 57, 17–25 (2017).
Somerset, D. A. et al. Ontogeny of hepatocyte growth factor (HGF) and its receptor (c-met) in human placenta: reduced HGF expression in intrauterine growth restriction. Am. J. Pathol. 153, 1139–1147 (1998).
Kauma, S., Hayes, N. & Weatherford, S. The differential expression of hepatocyte growth factor and met in human placenta. J. Clin. Endocrinol. Metab. 82, 949–954 (1997).
Herr, F., Schreiner, I., Baal, N., Pfarrer, C. & Zygmunt, M. Expression patterns of Notch receptors and their ligands Jagged and Delta in human placenta. Placenta 32, 554–563 (2011).
Haider, S. et al. Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc. Natl Acad. Sci. USA 113, E7710–E7719 (2016).
Wu, W.-X., Brooks, J., Glasier, A. F. & McNeilly, A. S. The relationship between decidualization and prolactin mRNA and production at different stages of human pregnancy. J. Mol. Endocrinol. 14, 255–261 (1995).
Stefanoska, I., Jovanović Krivokuća, M., Vasilijić, S., Ćujić, D. & Vićovac, L. Prolactin stimulates cell migration and invasion by human trophoblast in vitro. Placenta 34, 775–783 (2013).
Tuckey, R. C. Progesterone synthesis by the human placenta. Placenta 26, 273–281 (2005).
Wang, J. et al. Progesterone receptor immunoreactivity at the maternofetal interface of first trimester pregnancy: a study of the trophoblast population. Hum. Reprod. 11, 413–419 (1996).
Goldman, S. & Shalev, E. Difference in progesterone-receptor isoforms ratio between early and late first-trimester human trophoblast is associated with differential cell invasion and matrix metalloproteinase 2 expression. Biol. Reprod. 74, 13–22 (2006).
Meadows, J. W., Pitzer, B., Brockman, D. E. & Myatt, L. Differential localization of prostaglandin E synthase isoforms in human placental cell types. Placenta 25, 259–265 (2004).
Grigsby, P. L., Sooranna, S. R., Brockman, D. E., Johnson, M. R. & Myatt, L. Localization and expression of prostaglandin E2 receptors in human placenta and corresponding fetal membranes with labor. Am. J. Obstet. Gynecol. 195, 260–269 (2006).
Tilburgs, T. et al. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc. Natl Acad. Sci. USA. 112, 7219–7224 (2015).
Martinović, S. et al. Osteogenic protein-1 is produced by human fetal trophoblasts in vivo and regulates the synthesis of chorionic gonadotropin and progesterone by trophoblasts in vitro. Clin. Chem. Lab. Med. 34, 103 (1996).
Fournier, T., Guibourdenche, J. & Evain-Brion, D. Review: hCGs: different sources of production, different glycoforms and functions. Placenta 36, S60–S65 (2015).
Tao, Y.-X., Lei, Z. M., Hofmann, G. E. & Rao, C. V. Human intermediate trophoblasts express chorionic gonadotropin/luteinizing hormone receptor gene. Biol. Reprod. 53, 899–904 (1995).
Soliman, A. et al. Placental melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal Res. 59, 38–46 (2015).
Iwasaki, S. et al. Melatonin as a local regulator of human placental function. J. Pineal Res. 39, 261–265 (2005).
Butler, T. M., Pater, J. A. & MacPhee, D. J. Integrin linked kinase regulates syncytialization of BeWo trophoblast cells. Biol. Reprod. 96, 673–685 (2017).
Shiokawa, S. et al. Small guanosine triphospatase rhoa and rho-associated kinase as regulators of trophoblast migration. J. Clin. Endocrinol. Metab. 87, 5808–5816 (2002).
Burton, G. J., Yung, H. W. & Murray, A. J. Mitochondrial–endoplasmic reticulum interactions in the trophoblast: stress and senescence. Placenta 52, 146–155 (2017).
Li, L., Rubin, L. P. & Gong, X. MEF2 transcription factors in human placenta and involvement in cytotrophoblast invasion and differentiation. Physiol. Genomics 50, 10–19 (2018).
Turco, M. Y. et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 19, 568–577 (2017).
Fock, V. et al. Neuregulin-1-mediated ErbB2–ErbB3 signalling protects human trophoblasts against apoptosis to preserve differentiation. J. Cell Sci. 128, 4306 LP–4316 (2015).
Haider, S. et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 11, 537–551 (2018).
Margolis, L. & Sadovsky, Y. The biology of extracellular vesicles: the known unknowns. PLoS Biol. 17, e3000363–e3000363 (2019).
Moffett, A. & Colucci, F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol. Rev. 267, 283–297 (2015).
Burton, G. J., Jauniaux, E. & Charnock-Jones, D. S. The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 54, 303–312 (2010).
Hiby, S. E. et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965 (2004).
Mayhew, T. M. Turnover of human villous trophoblast in normal pregnancy: what do we know and what do we need to know? Placenta 35, 229–240 (2014).
Arnholdt, H., Meisel, F., Fandrey, K. & Löhrs, U. Proliferation of villous trophoblast of the human placenta in normal and abnormal pregnancies. Virchows Arch. B 60, 365–372 (1991).
Mansour, A. A. et al. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 36, 432–441 (2018).
Pham, M. T. et al. Generation of human vascularized brain organoids. Neuroreport 29, 588–593 (2018).
Wimmer, R. A. et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510 (2019).
Bar-Ephraim, Y. E., Kretzschmar, K. & Clevers, H. Organoids in immunological research. Nat. Rev. Immunol. 20, 279–293 (2020).
Co, J. Y. et al. Controlling epithelial polarity: a human enteroid model for host-pathogen interactions. Cell Rep. 26, 2509–2520.e4 (2019).
Abbas, Y. et al. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus 10, 20190079 (2020).
Cruz-Acuña, R. et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19, 1326–1335 (2017).
Apps, R. et al. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127, 26–39 (2009).
Acknowledgements
The authors are grateful to patients for donating tissue for research. We thank D. Moore and staff at Addenbrookes Hospital, Cambridge. This work was initiated and funded by the Centre for Trophoblast Research, University of Cambridge. G. J. Burton was funded by the Medical Research Council (MR/L020041/1). A. Moffett holds a Wellcome Trust joint investigator award with Dr F. Colucci (200841/Z/16/Z). M.Y. Turco received funding from the E.U. 7th Framework Programme for research, technological development and demonstration (PIEF-GA-2013-629785) and from the European Union’s Horizon 2020 research and innovation programme (Grant agreement no. [853546]). M.Y. Turco holds a Royal Society Dorothy Hodgkin Fellowship (DH160216) and a Royal Society Research Grant (RGF\R1\180028). We thank all members of the Moffett Laboratory for support.
Author information
Authors and Affiliations
Contributions
M.Y.T. and L.G. developed the organoid culture system for trophoblast in ref. 8. All the experiments undertaken while developing this protocol were carried out by M.A.S., R.C.F., L.G., and M.S.H. The manuscript was written by M.Y.T., M.A.S., L.G. and A.M. with help from R.C.F. and G.J.B. The figures were prepared by M.A.S., R.C.F., and M.S.H. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related Links
Key reference using this protocol
Turco, M. Y. et al. Nature 564, 263–267 (2018): https://doi.org/10.1038/s41586-018-0753-3
Supplementary information
Rights and permissions
About this article
Cite this article
Sheridan, M.A., Fernando, R.C., Gardner, L. et al. Establishment and differentiation of long-term trophoblast organoid cultures from the human placenta. Nat Protoc 15, 3441–3463 (2020). https://doi.org/10.1038/s41596-020-0381-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0381-x
This article is cited by
-
Single-nucleus multi-omic profiling of human placental syncytiotrophoblasts identifies cellular trajectories during pregnancy
Nature Genetics (2024)
-
Unraveling the molecular mechanisms driving enhanced invasion capability of extravillous trophoblast cells: a comprehensive review
Journal of Assisted Reproduction and Genetics (2024)
-
Single-cell characterization of self-renewing primary trophoblast organoids as modeling of EVT differentiation and interactions with decidual natural killer cells
BMC Genomics (2023)
-
A comprehensive review of human trophoblast fusion models: recent developments and challenges
Cell Death Discovery (2023)
-
Dynamic matrices with DNA-encoded viscoelasticity for cell and organoid culture
Nature Nanotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.