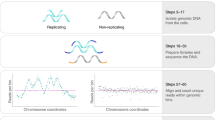
Abstract
Replication timing (RT) domains are stable units of chromosome structure that are regulated in the context of development and disease. Conventional genome-wide RT mapping methods require many S-phase cells for either the effective enrichment of replicating DNA through bromodeoxyuridine (BrdU) immunoprecipitation or the determination of copy-number differences during S-phase, which precludes their application to non-abundant cell types and single cells. Here, we provide a simple, cost-effective, and robust protocol for single-cell DNA replication sequencing (scRepli-seq). The scRepli-seq methodology relies on whole-genome amplification (WGA) of genomic DNA (gDNA) from single S-phase cells and next-generation sequencing (NGS)-based determination of copy-number differences that arise between replicated and unreplicated DNA. Haplotype-resolved scRepli-seq, which distinguishes pairs of homologous chromosomes within a single cell, is feasible by using single-nucleotide polymorphism (SNP)/indel information. We also provide computational pipelines for quality control, normalization, and binarization of the scRepli-seq data. The experimental portion of this protocol (before sequencing) takes 3 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The datasets used to generate the figures are deposited in the NCBI Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) under accession codes GSE138634, GSE108556, and GSE113985.
Code availability
The code used to analyze the scRepli-seq data in this study is available in Supplementary Software 1–4 and at https://github.com/kuzobuta/scRepliseq-Pipeline.
References
Berezney, R., Dubey, D. D. & Huberman, J. A. Heterogeneity of eukaryotic replicons, replicon clusters, and replication foci. Chromosoma 108, 471–484 (2000).
Hiratani, I. & Takahashi, S. DNA replication timing enters the single-cell era. Genes (Basel) 10, 221 (2019).
Rivera-Mulia, J. C. & Gilbert, D. M. Replication timing and transcriptional control: beyond cause and effect—part III. Curr. Opin. Cell Biol. 40, 168–178 (2016).
Fragkos, M., Ganier, O., Coulombe, P. & Méchali, M. DNA replication origin activation in space and time. Nat. Rev. Mol. Cell Biol. 16, 360–374 (2015).
Prioleau, M. N. & MacAlpine, D. M. DNA replication origins—where do we begin? Genes Dev. 30, 1683–1697 (2016).
Takebayashi, S. I., Ogata, M. & Okumura, K. Anatomy of mammalian replication domains. Genes (Basel) 8, 110 (2017).
Hiratani, I. et al. Genome-wide dynamics of replication timing revealed by in vitro models of mouse embryogenesis. Genome Res 20, 155–169 (2010).
Ryba, T. et al. Evolutionarily conserved replication timing profiles predict long-range chromatin interactions and distinguish closely related cell types. Genome Res 20, 761–770 (2010).
Dileep, V., Rivera-Mulia, J. C., Sima, J. & Gilbert, D. M. Large-scale chromatin structure-function relationships during the cell cycle and development: insights from replication timing. Cold Spring Harb. Symp. quant. Biol. 80, 53–63 (2016).
Pope, B. D. et al. Topologically associating domains are stable units of replication-timing regulation. Nature 515, 402–405 (2014).
Azuara, V. Profiling of DNA replication timing in unsynchronized cell populations. Nat. Protoc. 1, 2171–2177 (2006).
Hiratani, I. et al. Global reorganization of replication domains during embryonic stem cell differentiation. PLoS Biol. 6, 2220–2236 (2008).
Ryba, T., Battaglia, D., Pope, B. D., Hiratani, I. & Gilbert, D. M. Genome-scale analysis of replication timing: from bench to bioinformatics. Nat. Protoc. 6, 870–895 (2011).
Marchal, C. et al. Genome-wide analysis of replication timing by next-generation sequencing with E/L Repli-seq. Nat. Protoc. 13, 819–839 (2018).
Takebayashi, S. I., Ogata, S., Ogata, M. & Okumura, K. Mapping mammalian replication domains using the ion torrent semiconductor sequencing platform. Biosci. Biotechnol. Biochem. 82, 2098–2100 (2018).
Desprat, R. et al. Predictable dynamic program of timing of DNA replication in human cells. Genome Res. 19, 2288–2299 (2009).
Koren, A. & McCarroll, S. A. Random replication of the inactive X chromosome. Genome Res 24, 64–69 (2014).
Yehuda, Y. et al. Germline DNA replication timing shapes mammalian genome composition. Nucleic Acids Res 46, 8299–8310 (2018).
Selig, S., Okumura, K., Ward, D. C. & Cedar, H. Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO J. 11, 1217–1225 (1992).
Simon, I. et al. Asynchronous replication of imprinted genes is established in the gametes and maintained during development. Nature 401, 929–932 (1999).
Nogami, M. et al. Intranuclear arrangement of human chromosome 12 correlates to large-scale replication domains. Chromosoma 108, 514–522 (2000).
Azuara, V. et al. Heritable gene silencing in lymphocytes delays chromatid resolution without affecting the timing of DNA replication. Nat. Cell Biol. 5, 668–674 (2003).
Kitamura, E., Blow, J. J. & Tanaka, T. U. Live-cell imaging reveals replication of individual replicons in eukaryotic replication factories. Cell 125, 1297–1308 (2006).
Ebrahimi, H. et al. Early initiation of a replication origin tethered at the nuclear periphery. J. Cell Sci. 123, 1015–1019 (2010).
Saner, N. et al. Stochastic association of neighboring replicons creates replication factories in budding yeast. J. Cell Biol. 202, 1001–1012 (2013).
Duriez, B., Chilaka, S., Bercher, J.-F., Hercul, E. & Prioleau, M.-N. Replication dynamics of individual loci in single living cells reveal changes in the degree of replication stochasticity through S phase. Nucleic Acids Res. 47, 5155–5169 (2019).
Norio, P. et al. Progressive activation of DNA replication initiation in large domains of the immunoglobulin heavy chain locus during B cell development. Mol. Cell 20, 575–587 (2005).
Herrick, J. & Bensimon, A. Single molecule analysis of DNA replication. Biochimie 81, 859–871 (1999).
Lebofsky, R., Heilig, R., Sonnleitner, M., Weissenbach, J. & Bensimon, A. DNA replication origin interference increases the spacing between initiation events in human cells. Mol. Biol. Cell 17, 5337–5345 (2006).
Letessier, A. et al. Cell-type-specific replication initiation programs set fragility of the FRA3B fragile site. Nature 470, 120–124 (2011).
Hennion, M. et al. FORK-seq: replication landscape of the Saccharomyces cerevisiae genome by nanopore sequencing. Genome Biol. 21, 125 (2020).
Müller, C. A. et al. Capturing the dynamics of genome replication on individual ultra-long nanopore sequence reads. Nat. Methods 16, 429–436 (2019).
Lacroix, J. et al. Analysis of DNA replication by optical mapping in nanochannels. Small 12, 5963–5970 (2016).
Wang, W. et al. Genome-wide mapping of human DNA replication by optical replication mapping supports a stochastic model of eukaryotic replication timing. Preprint at bioRxiv https://doi.org/10.1101/2020.08.24.263459 (2020).
De Carli, F. et al. High-throughput optical mapping of replicating DNA. Small Methods 2, 1800146 (2018).
Takahashi, S. et al. Genome-wide stability of the DNA replication program in single mammalian cells. Nat. Genet. 51, 529–540 (2019).
Miura, H. et al. Single-cell DNA replication profiling identifies spatiotemporal developmental dynamics of chromosome organization. Nat. Genet. 51, 1356–1368 (2019).
Dileep, V. & Gilbert, D. M. Single-cell replication profiling to measure stochastic variation in mammalian replication timing. Nat. Commun. 9, 427 (2018).
Jackson, D. A. S-phase progression in synchronized human cells. Exp. Cell Res. 220, 62–70 (1995).
Snyder, A. R., Zhou, J., Deng, Z. & Lieberman, P. M. Therapeutic doses of hydroxyurea cause telomere dysfunction and reduce TRF2 binding to telomeres. Cancer Biol. Ther. 8, 1136–1145 (2009).
Courtot, L., Hoffmann, J.-S. & Bergoglio, V. The protective role of dormant origins in response to replicative stress. Int. J. Mol. Sci. 19, 3569 (2018).
Baslan, T. et al. Optimizing sparse sequencing of single cells for highly multiplex copy number profiling. Genome Res 125, 714–724 (2015).
Kadota, M. et al. CTCF binding landscape in jawless fish with reference to Hox cluster evolution. Sci. Rep. 7, 4957 (2017).
Li, H. & Durbin, R. Making the leap: Maq to BWA. Mass Genomics 25, 1754–1760 (2009).
Bakker, B. et al. Single-cell sequencing reveals karyotype heterogeneity in murine and human malignancies. Genome Biol. 17, 115 (2016).
Huang, L., Ma, F., Chapman, A., Lu, S. & Xie, X. S. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu. Rev. Genomics Hum. Genet. 16, 79–102 (2015).
Navin, N. E. Cancer genomics: one cell at a time. Genome Biol. 15, 452 (2014).
Spits, C. et al. Whole-genome multiple displacement amplification from single cells. Nat. Protoc. 1, 1965–1970 (2006).
Chen, C. et al. Single-cell whole-genome analyses by linear amplification via transposon insertion (LIANTI). Science 356, 189–194 (2017).
Baslan, T. et al. Genome-wide copy number analysis of single cells. Nat. Protoc. 7, 1024–1041 (2012).
Koberna, K. et al. Nuclear organization studied with the help of a hypotonic shift: its use permits hydrophilic molecules to enter into living cells. Chromosoma 108, 325–335 (1999).
Salic, A. & Mitchison, T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl Acad. Sci. USA 105, 2415–2420 (2008).
Takahashi, S., Kobayashi, S. & Hiratani, I. Epigenetic differences between naïve and primed pluripotent stem cells. Cell. Mol. Life Sci. 75, 1–13 (2017).
Saelens, W., Cannoodt, R., Todorov, H. & Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37, 547–554 (2019).
Dey, S. S., Kester, L., Spanjaard, B., Bienko, M. & Van Oudenaarden, A. Integrated genome and transcriptome sequencing of the same cell. Nat. Biotechnol. 33, 285–289 (2015).
Macaulay, I. C. et al. Separation and parallel sequencing of the genomes and transcriptomes of single cells using G&T-seq. Nat. Protoc. 11, 2081–2103 (2016).
Yamazaki, S. et al. Rif1 regulates the replication timing domains on the human genome. EMBO J. 31, 3667–3677 (2012).
Cornacchia, D. et al. Mouse Rif1 is a key regulator of the replication-timing programme in mammalian cells. EMBO J. 31, 3678–3690 (2012).
Adamson, B. et al. A multiplexed single-cell CRISPR screening platform enables systematic dissection of the unfolded protein response. Cell 167, 1867–1882.e21 (2016).
Dixit, A. et al. Perturb-seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167, 1853–1866.e17 (2016).
Jaitin, D. A. et al. Dissecting immune circuits by linking CRISPR-pooled screens with single-cell RNA-seq. Cell 167, 1883–1896.e15 (2016).
Datlinger, P. et al. Pooled CRISPR screening with single-cell transcriptome readout. Nat. Methods 14, 297–301 (2017).
Hayashi, K. & Saitou, M. Generation of eggs from mouse embryonic stem cells and induced pluripotent stem cells. Nat. Protoc. 8, 1513–1524 (2013).
Sasagawa, Y. et al. Quartz-seq: a highly reproducible and sensitive single-cell RNA sequencing method, reveals nongenetic gene-expression heterogeneity. Genome Biol. 14, 3097 (2013).
R Development Core Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, 2017). http://www.R-project.org.
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10 (2011).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Lassmann, T., Hayashizaki, Y. & Daub, C. O. SAMStat: monitoring biases in next generation sequencing data. Bioinformatics 27, 130–131 (2011).
Beaulieu-Jones, B. K. & Greene, C. S. Reproducibility of computational workflows is automated using continuous analysis. Nat. Biotechnol. 35, 342–346 (2017).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at https://arxiv.org/abs/arXiv:1303.3997 (2013).
Weinreb, C., Wolock, S. & Klein, A. M. SPRING: A kinetic interface for visualizing high dimensional single-cell expression data. Bioinformatics 34, 1246–1248 (2018).
Degner, J. F. et al. Effect of read-mapping biases on detecting allele-specific expression from RNA-sequencing data. Bioinformatics 25, 3207–3212 (2009).
Manders, E. M. M., Kimura, H. & Cook, P. R. Direct imaging of DNA in living cells reveals the dynamics of chromosome formation. J. Cell Biol. 144, 813–821 (1999).
Dimitrova, D. S. & Berezney, R. The spatio-temporal organization of DNA replication sites is identical in primary, immortalized and transformed mammalian cells. J. Cell Sci. 115, 4037–4051 (2002).
Wu, R., Terry, A. V., Singh, P. B. & Gilbert, D. M. Differential subnuclear localization and replication timing of histone H3 lysine 9 methylation states. Mol. Biol. Cell 16, 2872–2881 (2005).
Kuriya, K. et al. Direct visualization of DNA replication dynamics in zebrafish cells. Zebrafish 12, 432–439 (2015).
Wutz, A. Haploid animal cells. Development 141, 1423–1426 (2014).
Kuhn, R. M., Haussler, D. & James Kent, W. The UCSC Genome Browser and associated tools. Brief. Bioinform. 14, 144–161 (2013).
Krueger, F. & Andrews, S. R. SNPsplit: allele-specific splitting of alignments between genomes with known SNP genotypes. F1000Res. 5, 1479 (2016).
Sakata, Y. et al. Defects in dosage compensation impact global gene regulation in the mouse trophoblast. Development 144, 2784–2797 (2017).
Acknowledgements
We thank the Center for Molecular Biology and Genetics of Mie University for NGS services. We also thank S. Kuraku and members of his laboratory for assistance with NGS, F. Matsuzaki for the use of the FACS instrument, A. Tanigawa and Y. Kondo for technical assistance, S-i. Hiraga for technical assistance in the Docker analysis and helpful comments, and L. Choubani for helpful comments. This work was supported by a RIKEN CDB/BDR intramural grant to I.H.; an award from the Special Postdoctoral Researcher (SPDR) Program of RIKEN to S.T.; an award from the RIKEN ‘Epigenome Manipulation Project’ of the All-RIKEN Projects to I.H.; MEXT KAKENHI grants JP16H01405 (to S.-i.T.), JP18H05530 (to I.H.), and JP15H01462 and JP17H06426 (to K.N.); and JSPS KAKENHI grants JP19K06610 (to S.-i.T.), JP18K14681 (to S.T.), and JP15K06942 (to K.N.).
Author information
Authors and Affiliations
Contributions
H.M., S.T., T.S., I.H., and S.-i.T. conceived the project. S.T., T.S., and S.-i.T. developed and conducted scRepli-seq. H.M. established the scRepli-seq data analysis pipeline. S.T., T.S., I.H. and S.-i.T. performed cell culture and sample collection. K.N. and C.O. constructed a diploid reference genome and helped with the haplotype-resolved analysis pipeline setup. K.O. and M.O. supported the design and execution of the project. H.M., S.T., I.H. and S.-i.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Chongyi Chen, Marie-Noëlle Prioleau and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Takahashi, S. et al. Nat. Genet. 51, 529–540 (2019): https://doi.org/10.1038/s41588-019-0347-5
Miura, H. et al. Nat. Genet. 51, 1356–1368 (2019): https://doi.org/10.1038/s41588-019-0474-z
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Supplementary Note.
Supplementary Software 1
Supplementary Software 1
Supplementary Software 2
Supplementary Software 2
Supplementary Software 3
Supplementary Software 3
Supplementary Software 4
Supplementary Software 4
Rights and permissions
About this article
Cite this article
Miura, H., Takahashi, S., Shibata, T. et al. Mapping replication timing domains genome wide in single mammalian cells with single-cell DNA replication sequencing. Nat Protoc 15, 4058–4100 (2020). https://doi.org/10.1038/s41596-020-0378-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0378-5
This article is cited by
-
Replication dynamics identifies the folding principles of the inactive X chromosome
Nature Structural & Molecular Biology (2023)
-
Optimized Repli-seq: improved DNA replication timing analysis by next-generation sequencing
Chromosome Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.