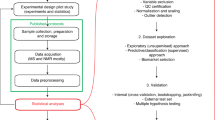
Abstract
Metabolic profiling of biological samples provides important insights into multiple physiological and pathological processes but is hindered by a lack of automated annotation and standardized methods for structure elucidation of candidate disease biomarkers. Here we describe a system for identifying molecular species derived from nuclear magnetic resonance (NMR) spectroscopy-based metabolic phenotyping studies, with detailed information on sample preparation, data acquisition and data modeling. We provide eight different modular workflows to be followed in a recommended sequential order according to their level of difficulty. This multi-platform system involves the use of statistical spectroscopic tools such as Statistical Total Correlation Spectroscopy (STOCSY), Subset Optimization by Reference Matching (STORM) and Resolution-Enhanced (RED)-STORM to identify other signals in the NMR spectra relating to the same molecule. It also uses two-dimensional NMR spectroscopic analysis, separation and pre-concentration techniques, multiple hyphenated analytical platforms and data extraction from existing databases. The complete system, using all eight workflows, would take up to a month, as it includes multi-dimensional NMR experiments that require prolonged experiment times. However, easier identification cases using fewer steps would take 2 or 3 days. This approach to biomarker discovery is efficient and cost-effective and offers increased chemical space coverage of the metabolome, resulting in faster and more accurate assignment of NMR-generated biomarkers arising from metabolic phenotyping studies. It requires a basic understanding of MATLAB to use the statistical spectroscopic tools and analytical skills to perform solid phase extraction (SPE), liquid chromatography (LC) fraction collection, LC-NMR-mass spectroscopy and one-dimensional and two-dimensional NMR experiments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Code availability
CA-PLS (and PLS, OSC-PLS): The code for executing the PLS, covariate-adjusted (O)PLS and simple orthogonal PLS/PLS-DA is provided in https://bitbucket.org/jmp111/capls/src/. This can be executed in a MATLAB environment.
STORM (and STOCSY): The code for executing both the STOCSY and STORM algorithms is provided in https://bitbucket.org/jmp111/storm/src. These can be executed in a MATLAB environment.
RED-STORM: The code for executing the RED-STORM algorithm is provided in https://bitbucket.org/jmp111/redstorm/src/. This can be executed in a MATLAB environment.
References
Holmes, E., Wilson, I. D. & Nicholson, J. K. Metabolic phenotyping in health and disease. Cell 134, 714–717 (2008).
Nicholson, J. K., Lindon, J. C. & Holmes, E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29, 1181–1189 (1999).
Fiehn, O. Metabolomics - the link between genotypes and phenotypes. Plant Mol. Biol. 48, 155–171 (2002).
Nicholson, J. K. & Wilson, I. D. High-resolution proton magnetic-resonance spectroscopy of biological-fluids. Prog. Nucl. Mag. Res. Spectr. 21, 449–501 (1989).
Nicholson, J. K. et al. Proton-nuclear-magnetic-resonance studies of serum, plasma and urine from fasting normal and diabetic subjects. Biochem. J. 217, 365–375 (1984).
Bales, J. R., Higham, D. P., Howe, I., Nicholson, J. K. & Sadler, P. J. Use of high-resolution proton nuclear magnetic resonance spectroscopy for rapid multi-component analysis of urine. Clin. Chem. 30, 426–432 (1984).
Wilson, I. D., Wade, K. E. & Nicholson, J. K. Analysis of biological-fluids by high-field nuclear magnetic-resonance spectroscopy. Trac Trend Anal. Chem. 8, 368–374 (1989).
Belton, P. S. et al. Use of high-field H-1 NMR spectroscopy for the analysis of liquid foods. J. Agric. Food Chem. 44, 1483–1487 (1996).
Cloarec, O. et al. Statistical total correlation spectroscopy: an exploratory approach for latent biomarker identification from metabolic H-1 NMR data sets. Anal. Chem. 77, 1282–1289 (2005).
Posma, J. M. et al. Subset optimization by reference matching (STORM): an optimized statistical approach for recovery of metabolic biomarker structural information from 1H NMR spectra of biofluids. Anal. Chem. 84, 10694–10701 (2012).
Posma, J. M. et al. Integrated analytical and statistical two-dimensional spectroscopy strategy for metabolite identification: application to dietary biomarkers. Anal. Chem. 89, 3300–3309 (2017).
Nicholson, J. K., Foxall, P. J. D., Spraul, M., Farrant, R. D. & Lindon, J. C. 750-Mhz H-1 and H-1-C-13 Nmr-spectroscopy of human blood-plasma. Anal. Chem. 67, 793–811 (1995).
Beckonert, O. et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2, 2692–2703 (2007).
Dona, A. C. et al. A guide to the identification of metabolites in NMR-based metabonomics/metabolomics experiments. Comput. Struct. Biotechnol. J. 14, 135–153 (2016).
Godejohann, M., Tseng, L. H., Braumann, U., Fuchser, J. & Spraul, M. Characterization of a paracetamol metabolite using on-line LC-SPE-NMR-MS and a cryogenic NMR probe. J. Chromatogr. A 1058, 191–196 (2004).
Shockcor, J. P. et al. Combined HPLC, NMR spectroscopy, and ion-trap mass spectrometry with application to the detection and characterization of xenobiotic and endogenous metabolites in human urine. Anal. Chem. 68, 4431–4435 (1996).
Coles, S. J., Day, N. E., Murray-Rust, P., Rzepa, H. S. & Zhang, Y. Enhancement of the chemical semantic web through the use of InChI identifiers. Org. Biomol. Chem. 3, 1832–1834 (2005).
Sumner, L. W. et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3, 211–221 (2007).
Wishart, D. S. Computational strategies for metabolite identification in metabolomics. Bioanalysis 1, 1579–1596 (2009).
Ellinger, J. J., Chylla, R. A., Ulrich, E. L. & Markley, J. L. Databases and software for NMR-based metabolomics. Curr. Metabol. https://doi.org/10.2174/2213235X11301010028 (2013).
Wishart, D. S. et al. HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 37, D603–D610 (2009).
Ulrich, E. L. et al. BioMagResBank. Nucleic Acids Res. 36, D402–D408 (2008).
Akiyama, K. et al. PRIMe: a Web site that assembles tools for metabolomics and transcriptomics. Silico Biol. 8, 339–345 (2008).
Wishart, D. S. Quantitative metabolomics using NMR. Trac Trend Anal. Chem. 27, 228–237 (2008).
Simpson, A. J., McNally, D. J. & Simpson, M. J. NMR spectroscopy in environmental research: from molecular interactions to global processes. Prog. Nucl. Magn. Reson. Spectr. 58, 97–175 (2011).
Dalisay, D. S. & Molinski, T. F. NMR quantitation of natural products at the nanomole scale. J. Nat. Prod. 72, 739–744 (2009).
Dona, A. C. et al. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 86, 9887–9894 (2014).
Kumar, D. Nuclear magnetic resonance (NMR) spectroscopy for metabolic profiling of medicinal plants and their products. Crit. Rev. Anal. Chem. 46, 400–412 (2016).
Fonville, J. M. et al. Evaluation of full-resolution J-resolved 1H NMR projections of biofluids for metabonomics information retrieval and biomarker identification. Anal. Chem. 82, 1811–1821 (2010).
Ludwig, C. & Viant, M. R. Two-dimensional J-resolved NMR spectroscopy: review of a key methodology in the metabolomics toolbox. Phytochem. Anal. 21, 22–32 (2010).
Viant, M. R. Improved methods for the acquisition and interpretation of NMR metabolomic data. Biochem. Biophys. Res. Commun. 310, 943–948 (2003).
Foxall, P. J. D., Parkinson, J. A., Sadler, I. H., Lindon, J. C. & Nicholson, J. K. Analysis of biological-fluids using 600 Mhz proton Nmr-spectroscopy - application of homonuclear 2-dimensional J-resolved spectroscopy to urine and blood-plasma for spectral simplification and assignment. J. Pharm. Biomed. 11, 21–31 (1993).
Liu, M., Nicholson, J. K. & Lindon, J. C. High-resolution diffusion and relaxation edited one- and two-dimensional 1H NMR spectroscopy of biological fluids. Anal. Chem. 68, 3370–3376 (1996).
Spraul, M., Nicholson, J. K., Lynch, M. J. & Lindon, J. C. Application of the one-dimensional Tocsy pulse sequence in 750 Mhz H-1-Nmr spectroscopy for assignment of endogenous metabolite resonances in biofluids. J. Pharm. Biomed. 12, 613–618 (1994).
Lindon, J. C., Nicholson, J. K. & Wilson, I. D. Directly coupled HPLC-NMR and HPLC-NMR-MS in pharmaceutical research and development. J. Chromatogr. B 748, 233–258 (2000).
Noda, I. Generalized 2-dimensional correlation method applicable to infrared, Raman, and other types of spectroscopy. Appl. Spectrosc. 47, 1329–1336 (1993).
Robinette, S. L., Lindon, J. C. & Nicholson, J. K. Statistical spectroscopic tools for biomarker discovery and systems medicine. Anal. Chem. 85, 5297–5303 (2013).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B Met. 57, 289–300 (1995).
Storey, J. D. & Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl Acad. Sci. USA 100, 9440–9445 (2003).
Elliott, P. et al. Urinary metabolic signatures of human adiposity. Sci. Transl. Med. 7, 285ra262 (2015).
Garcia-Perez, I. et al. An analytical pipeline for quantitative characterization of dietary intake: application to assess grape intake. J. Agric. Food Chem. 64, 2423–2431 (2016).
Garcia-Perez, I. et al. Bidirectional correlation of NMR and capillary electrophoresis fingerprints: a new approach to investigating Schistosoma mansoni infection in a mouse model. Anal. Chem. 82, 203–210 (2010).
Garcia-Perez, I. et al. Urinary metabolic phenotyping the slc26a6 (chloride-oxalate exchanger) null mouse model. J. Proteome Res. 11, 4425–4435 (2012).
Andreas, N. J. et al. Multiplatform characterization of dynamic changes in breast milk during lactation. Electrophoresis 36, 2269–2285 (2015).
Garcia-Perez, I. et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endo. 5, 184–195 (2017).
Posma, J. M. et al. Optimized phenotypic biomarker discovery and confounder elimination via covariate-adjusted projection to latent structures from metabolic spectroscopy data. J. Proteome Res. 17, 1586–1595 (2018).
Trygg, J., Holmes, E. & Lundstedt, T. Chemometrics in metabonomics. J. Proteome Res. 6, 469–479 (2007).
Baranovicova, E. et al. NMR metabolomic study of blood plasma in ischemic and ischemically preconditioned rats: an increased level of ketone bodies and decreased content of glycolytic products 24 h after global cerebral ischemia. J. Physiol. Biochem. https://doi.org/10.1007/s13105-018-0632-2 (2018).
Scott, I. M. et al. Merits of random forests emerge in evaluation of chemometric classifiers by external validation. Anal. Chim. Acta 801, 22–33 (2013).
Cavill, R. et al. Genetic algorithms for simultaneous variable and sample selection in metabonomics. Bioinformatics 25, 112–118 (2009).
Di Anibal, C. V., Callao, M. P. & Ruisanchez, I. 1H NMR variable selection approaches for classification. A case study: the determination of adulterated foodstuffs. Talanta 86, 316–323 (2011).
Wang, T. et al. Automics: an integrated platform for NMR-based metabonomics spectral processing and data analysis. BMC Bioinforma. 10, 83 (2009).
Balabin, R. M., Safieva, R. Z. & Lomakina, E. I. Gasoline classification using near infrared (NIR) spectroscopy data: comparison of multivariate techniques. Anal. Chim. Acta 671, 27–35 (2010).
Tiwari, P., Rosen, M. & Madabhushi, A. A hierarchical spectral clustering and nonlinear dimensionality reduction scheme for detection of prostate cancer from magnetic resonance spectroscopy (MRS). Med. Phys. 36, 3927–3939 (2009).
Fotiou, M. et al. (1)H NMR-based metabolomics reveals the effect of maternal habitual dietary patterns on human amniotic fluid profile. Sci. Rep. 8, 4076 (2018).
Holmes, E., Cloarec, O. & Nicholson, J. K. Probing latent biomarker signatures and in vivo pathway activity in experimental disease states via statistical total correlation spectroscopy (STOCSY) of biofluids: application to HgCl2 toxicity. J. Proteome Res. 5, 1313––1320 (2006).
Alves, A. C., Rantalainen, M., Holmes, E., Nicholson, J. K. & Ebbels, T. M. Analytic properties of statistical total correlation spectroscopy based information recovery in 1H NMR metabolic data sets. Anal. Chem. 81, 2075–2084 (2009).
Rodriguez-Martinez, A., Ayala, R., Posma, J. M. & Dumas, M. E. Exploring the genetic landscape of metabolic phenotypes with metaboSignal. Curr. Protoc. Bioinform. 61, 14 14 11–14 14 13 (2018).
Wang, Y. et al. Magic angle spinning NMR and 1H-31P heteronuclear statistical total correlation spectroscopy of intact human gut biopsies. Anal. Chem. 80, 1058–1066 (2008).
Keun, H. C. et al. Heteronuclear F-19-H-1 statistical total correlation spectroscopy as a tool in drug metabolism: Study of flucloxacillin biotransformation. Anal. Chem. 80, 1073–1079 (2008).
Aue, W. P., Karhan, J. & Ernst, R. R. Homonuclear broad-band decoupling and 2-dimensional J-resolved Nmr-spectroscopy. J. Chem. Phys. 64, 4226–4227 (1976).
Nagayama, K., Kumar, A., Wuthrich, K. & Ernst, R. R. Experimental-techniques of two-dimensional correlated spectroscopy. J. Magn. Reson. 40, 321–334 (1980).
Aue, W. P., Bartholdi, E. & Ernst, R. R. 2-Dimensional spectroscopy - application to nuclear magnetic-resonance. J. Chem. Phys. 64, 2229–2246 (1976).
Bodenhausen, G. & Ruben, D. J. Natural abundance N-15 Nmr by enhanced heteronuclear spectroscopy. Chem. Phys. Lett. 69, 185–189 (1980).
Keeler, J. Understanding NMR Spectroscopy 2nd edn (John Wiley & Sons, 2002).
Bax, A., Farley, K. A. & Walker, G. S. Increased HMBC sensitivity for correlating poorly resolved proton multiplets to carbon-13 using selective or semi-selective pulses. J. Magn. Reson. Ser. A 119, 134–138 (1996).
Bollard, M. E. et al. High-resolution (1)H and (1)H-(13)C magic angle spinning NMR spectroscopy of rat liver. Magn. Reson. Med. 44, 201–207 (2000).
Smith, L. M. et al. Statistical correlation and projection methods for improved information recovery from diffusion-edited NMR spectra of biological samples. Anal. Chem. 79, 5682–5689 (2007).
Tang, H. R., Wang, Y. L., Nicholson, J. K. & Lindon, J. C. Use of relaxation-edited one-dimensional and two dimensional nuclear magnetic resonance spectroscopy to improve detection of small metabolites in blood plasma. Anal. Biochem. 325, 260–272 (2004).
Lenz, E. M. Nuclear magnetic resonance (NMR)-based drug metabolite profiling. Methods Mol. Biol. 708, 299–319 (2011).
Ramautar, R., Somsen, G. W. & de Jong, G. J. CE-MS in metabolomics. Electrophoresis 30, 276–291 (2009).
Garcia-Perez, I. et al. Metabolic fingerprinting of Schistosoma mansoni infection in mice urine with capillary electrophoresis. Electrophoresis 29, 3201–3206 (2008).
Fiehn, O. Metabolomics by gas chromatography-mass spectrometry: combined targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 114, 30 34 31–30 34 32 (2016).
Spraul, M., Nicholson, J. K., Lynch, M. J. & Lindon, J. C. Application of the one-dimensional TOCSY pulse sequence in 750 MHz 1H-NMR spectroscopy for assignment of endogenous metabolite resonances in biofluids. J. Pharm. Biomed. Anal. 12, 613–618 (1994).
Crockford, D. J. et al. Statistical heterospectroscopy, an approach to the integrated analysis of NMR and UPLC-MS data sets: application in metabonomic toxicology studies. Anal. Chem. 78, 363–371 (2006).
Teul, J. et al. Improving metabolite knowledge in stable atherosclerosis patients by association and correlation of GC-MS and 1H NMR fingerprints. J. Proteome Res. 8, 5580–5589 (2009).
Posma, J. M., Robinette, S. L., Holmes, E. & Nicholson, J. K. MetaboNetworks, an interactive Matlab-based toolbox for creating, customizing and exploring sub-networks from KEGG. Bioinformatics 30, 893–895 (2014).
Quinn, R. A. et al. Molecular networking as a drug discovery, drug metabolism, and precision medicine strategy. Trends Pharmacol. Sci. 38, 143–154 (2017).
Gratton, J. et al. Optimized sample handling strategy for metabolic profiling of human feces. Anal. Chem. 88, 4661–4668 (2016).
Farrant, R. D., Lindon, J. C. & Nicholson, J. K. Internal temperature calibration for 1H NMR spectroscopy studies of blood plasma and other biofluids. NMR Biomed. 7, 243–247 (1994).
Holmes, E. et al. 750 MHz 1H NMR spectroscopy characterisation of the complex metabolic pattern of urine from patients with inborn errors of metabolism: 2-hydroxyglutaric aciduria and maple syrup urine disease. J. Pharm. Biomed. Anal. 15, 1647–1659 (1997).
Duarte, I. F. et al. Identification of metabolites in human hepatic bile using 800 MHz 1H NMR spectroscopy, HPLC-NMR/MS and UPLC-MS. Mol. Biosyst. 5, 180–190 (2009).
Maaheimo, H., Rabina, J. & Renkonen, O. 1H and 13C NMR analysis of the pentasaccharide Gal beta (1->4)GlcNAc beta (1->3)-[GlcNAc beta (1->6)]Gal beta (1->4)GlcNAc synthesized by the mid-chain beta-(1->6)-D-N-acetylglucosaminyltransferase of rat serum. Carbohydr. Res. 297, 145–151 (1997).
Want, E. J. et al. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 5, 1005–1018 (2010).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Tredwell, G. D., Bundy, J. G., De Iorio, M. & Ebbels, T. M. Modelling the acid/base (1)H NMR chemical shift limits of metabolites in human urine. Metabolomics 12, 152 (2016).
Acknowledgements
This article presents independent research funded by the UK National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the UK National Health Service (NHS), the NIHR or the UK Department of Health. I.G.-P. is supported by a National Institute for Health Research (NIHR) fellowship (NIHR-CDF-2017-10-032). J.M.P. is supported by a Rutherford Fund Fellowship at Health Data Research UK (MR/S004033/1). G.F. is an NIHR Senior Investigator. P.E. is Director of the Medical Research Council (MRC) Centre for Environment and Health (MR/L01341X/1) and acknowledges support from the NIHR Imperial Biomedical Research Centre and the NIHR Health Protection Research Unit in Health Impact of Environmental Hazards (HPRU-2012-10141). P.E. is supported by the UK Dementia Research Institute, supported by UK DRI Ltd., which is funded by the UK MRC, the Alzheimer’s Society and Alzheimer’s Research UK. INTERMAP is supported by the US National Heart, Lung and Blood Institute (grants R01-HL050490 and R01-HL084228), the Chicago Health Research Foundation and national agencies in Japan (grant [A] 090357003) and the UK (R2019EPH). Infrastructure support was provided by the NIHR Imperial Biomedical Research Centre and the UK MEDical BIOinformatics partnership (MR/L01632X/1). I.G.-P. gratefully acknowledges Olaf Beckonert for his guidance. J.K.N. acknowledges the Australian Government Future Food Systems Cooperative Research Centre (CRC). E.H. is supported by the Department of Jobs, Tourism, Science and Innovation, Government of Western Australian, through the Premier’s Science Fellowship Program.
Author information
Authors and Affiliations
Contributions
Writing—review and editing: I.G.-P., J.M.P., J.L., I.S.C., J.S., G.F., P.E., E.H. and J.K.N. Contributed data: P.E., Q.C., C.L.B. and I.G.-P. Figures and tables: I.G.-P. and C.L.B. Sample analysis: I.G.-P. Statistical analysis and software development: J.M.P. Protocol and workflow design: I.G.-P. Funding acquisition: J.M.P., I.G.-P., E.H., P.E. and J.K.N.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Rob Verpoorte and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Elliott, P. et al. Sci. Transl. Med. 7, 285ra262 (2015): https://doi.org/10.1126/scitranslmed.aaa5680
Posma, J. M. et al. Anal. Chem. 89, 3300−3309 (2017): https://doi.org/10.1021/acs.analchem.6b03324
Garcia-Perez, I. et al. Lancet Diabet. Endo. 5, 184−195 (2017): https://doi.org/10.1016/S2213-8587(16)30419-3
Key data used in this protocol
Orbán-Németh, Z. et al. Nat. Protoc. 13, 478−494 (2018): https://doi.org/10.1038/nprot.2017.146
Supplementary information
Rights and permissions
About this article
Cite this article
Garcia-Perez, I., Posma, J.M., Serrano-Contreras, J.I. et al. Identifying unknown metabolites using NMR-based metabolic profiling techniques. Nat Protoc 15, 2538–2567 (2020). https://doi.org/10.1038/s41596-020-0343-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0343-3
This article is cited by
-
Small molecule metabolites: discovery of biomarkers and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Identification of metabolites from complex mixtures by 3D correlation of 1H NMR, MS and LC data using the SCORE-metabolite-ID approach
Scientific Reports (2023)
-
Leaf tissue metabolomics fingerprinting of Citronella gongonha Mart. by 1H HR-MAS NMR
Scientific Reports (2022)
-
Problems, principles and progress in computational annotation of NMR metabolomics data
Metabolomics (2022)
-
Natural products in drug discovery: advances and opportunities
Nature Reviews Drug Discovery (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.