Abstract
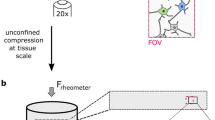
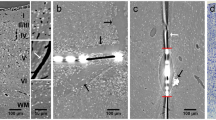
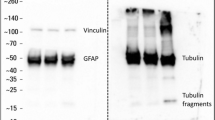
We recently developed an in vivo compression device that simulates the solid mechanical forces exerted by a growing tumor on the surrounding brain tissue and delineates the physical versus biological effects of a tumor. This device, to our knowledge the first of its kind, can recapitulate the compressive forces on the cerebellar cortex from primary (e.g., glioblastoma) and metastatic (e.g., breast cancer) tumors, as well as on the cerebellum from tumors such as medulloblastoma and ependymoma. We adapted standard transparent cranial windows normally used for intravital imaging studies in mice to include a turnable screw for controlled compression (acute or chronic) and decompression of the cerebral cortex. The device enables longitudinal imaging of the compressed brain tissue over several weeks or months as the screw is progressively extended against the brain tissue to recapitulate tumor growth–induced solid stress. The cranial window can be simply installed on the mouse skull according to previously established methods, and the screw mechanism can be readily manufactured in-house. The total time for construction and implantation of the in vivo compressive cranial window is <1 h (per mouse). This technique can also be used to study a variety of other diseases or disorders that present with abnormal solid masses in the brain, including cysts and benign growths.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
Code availability
The code associated with the datasets generated and/or analyzed during the current study is available from the corresponding author upon request.
References
Jain, R. K., Martin, J. D. & Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346 (2014).
Mitchell, M. J., Jain, R. K. & Langer, R. Engineering and physical sciences in oncology: challenges and opportunities. Nat. Rev. Cancer 17, 659–675 (2017).
Helmlinger, G. et al. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 15, 778–783 (1997).
Stylianopoulos, T. et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl Acad. Sci. USA 109, 15101–15108 (2012).
Nia, H. T. et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng. 1, 0004 (2016).
Padera, T. P. et al. Pathology: cancer cells compress intratumour vessels. Nature 427, 695 (2004).
Chauhan, V. P. et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4, 2516 (2013).
Chauhan, V. P. et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 26, 14–15 (2014).
Tse, J. M. et al. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl Acad. Sci. USA 109, 911–916 (2012).
Ricca, B. L. et al. Transient external force induces phenotypic reversion of malignant epithelial structures via nitric oxide signaling. Elife 7, e26161 (2018).
Montel, F. et al. Stress clamp experiments on multicellular tumor spheroids. Phys. Rev. Lett. 107, 188102 (2011).
Delarue, M. et al. Compressive stress inhibits proliferation in tumor spheroids through a volume limitation. Biophys. J. 107, 1821–1828 (2014).
Fernández-Sánchez, M. E. et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523, 92–95 (2015).
Seano, G. et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng. 3, 230–245 (2019).
Nia, H. T. et al. Quantifying solid stress and elastic energy from excised or in situ tumors. Nat. Protoc. 13, 1091–1105 (2018).
Amidei, C. & Kushner, D. S. Clinical implications of motor deficits related to brain tumors. Neurooncol. Prac. 2, 179–184 (2015).
Mukand, J. A. et al. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am. J. Phys. Med. Rehabil. 80, 346–350 (2001).
Kushner, D. S. & Amidei, C. Rehabilitation of motor dysfunction in primary brain tumor patients. Neurooncol. Prac. 2, 185–191 (2015).
Sawaya, R. et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42, 1044–1055 (1998). discussion 1055-1056.
Rees, J. H. Diagnosis and treatment in neuro-oncology: an oncological perspective. Br. J. Radiol. 84(Spec. No. 2), S82–S89 (2011).
Farago, N. et al. Human neuronal changes in brain edema and increased intracranial pressure. Acta Neuropathol. Commun. 4, 78 (2016).
Goriely, A. et al. Mechanics of the brain: perspectives, challenges, and opportunities. Biomech. Model. Mechanobiol. 14, 931–965 (2015).
Unterberg, A. W. et al. Edema and brain trauma. Neuroscience 129, 1021–1029 (2004).
de Groot, J. & Sontheimer, H. Glutamate and the biology of gliomas. Glia 59, 1181–1189 (2011).
Sontheimer, H. A role for glutamate in growth and invasion of primary brain tumors. J. Neurochem. 105, 287–295 (2008).
Savaskan, N. E. et al. Neurodegeneration in the brain tumor microenvironment: glutamate in the limelight. Curr. Neuropharmacol. 13, 258–265 (2015).
Huisman, T. A. Tumor-like lesions of the brain. Cancer Imaging 9(Special Issue A), S10–S13 (2009).
Cunliffe, C. H. et al. Intracranial lesions mimicking neoplasms. Arch. Pathol. Lab. Med. 133, 101–123 (2009).
Askoxylakis, V. et al. A cerebellar window for intravital imaging of normal and disease states in mice. Nat. Protoc. 12, 2251–2262 (2017).
Snuderl, M. et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 152, 1065–1076 (2013).
Bar-Kochba, E. et al. Strain and rate-dependent neuronal injury in a 3D in vitro compression model of traumatic brain injury. Sci. Rep. 6, 30550 (2016).
Lusardi, T. A. et al. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J. Neurotrauma 21, 61–72 (2004).
Pfister, B. J. et al. An in vitro uniaxial stretch model for axonal injury. Ann. Biomed. Eng. 31, 589–598 (2003).
Teixeira, F. G. et al. Bioengineered cell culture systems of central nervous system injury and disease. Drug Discov. Today 21, 1456–1463 (2016).
Schoeler, M. et al. Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury. BMC Neurol. 12, 20 (2012).
Morrison, B. III et al. An in vitro model of traumatic brain injury utilising two-dimensional stretch of organotypic hippocampal slice cultures. J. Neurosci. Methods 150, 192–201 (2006).
Xu, B. N. et al. Pathophysiology of brain swelling after acute experimental brain compression and decompression. Neurosurgery 32, 289–296 (1993). discussion 296.
Miller, J. D., Stanek, A. E. & Langfitt, T. W. Cerebral blood flow regulation during experimental brain compression. J. Neurosurg. 39, 186–196 (1973).
Leech, P. & Miller, J. D. Intracranial volume–pressure relationships during experimental brain compression in primates: 1. Pressure responses to changes in ventricular volume. J. Neurol. Neurosurg. Psychiatry 37, 1093–1098 (1974).
De la Torre, J. C. et al. Dimethyl sulfoxide in the treatment of experimental brain compression. J. Neurosurg. 38, 345–354 (1973).
Sullivan, H. G. et al. The physiological basis of intracranial pressure change with progressive epidural brain compression. An experimental evaluation in cats. J. Neurosurg. 47, 532–550 (1977).
Schettini, A. & Walsh, E. K. Brain tissue elastic behavior and experimental brain compression. Am. J. Physiol. 255(5 Pt 2), R799–R805 (1988).
Schettini, A. & Walsh, E. K. Brain elastic behavior in experimental brain compression: influence of steroid therapy. Brain Res. 305, 141–143 (1984).
Calabrese, E. et al. A diffusion MRI tractography connectome of the mouse brain and comparison with neuronal tracer data. Cereb. Cortex 25, 4628–4637 (2015).
Kober, F., Duhamel, G. & Callot, V. Cerebral perfusion MRI in mice, in In Vivo NMR Imaging 117-138 (Springer, New York, 2011).
Kearney, S. P. et al. Simultaneous 3D MR elastography of the in vivo mouse brain. Phys. Med. Biol. 62, 7682–7693 (2017).
Kamoun, W. S. et al. Simultaneous measurement of RBC velocity, flux, hematocrit and shear rate in vascular networks. Nat. Methods 7, 655–660 (2010).
Fukumura, D. et al. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17, 206–225 (2010).
Jain, R. K., Munn, L. L. & Fukumura, D. Dissecting tumour pathophysiology using intravital microscopy. Nat. Rev. Cancer 2, 266–276 (2002).
Blatter, C. et al. Simultaneous measurements of lymphatic vessel contraction, flow and valve dynamics in multiple lymphangions using optical coherence tomography. J. Biophotonics Vol. 11, e201700017 (2018).
Blatter, C. et al. In vivo label-free measurement of lymph flow velocity and volumetric flow rates using Doppler optical coherence tomography. Sci. Rep. 6, 29035 (2016).
Vakoc, B. J. et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 15, 1219–1223 (2009).
Kamoun, W. S. et al. Edema control by cediranib, a vascular endothelial growth factor receptor–targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J. Clin. Oncol. 27, 2542–2552 (2009).
Boucher, Y. et al. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br. J. Cancer 75, 829–836 (1997).
Brooks, S. P., Trueman, R. C. & Dunnett, S. B. Assessment of motor coordination and balance in mice using the Rotarod, elevated bridge, and footprint tests. Curr. Protoc. Mouse Biol. 2, 37–53 (2012).
Arvanitis, C. D. et al. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood–tumor barrier disruption. Proc. Natl Acad. Sci. USA 115, e8717–e8726 (2018).
Dong, H. W. The Allen Reference Atlas: A Digital Color Brain Atlas of the C57Bl/6J Male Mouse ix, 366 (Wiley, 2008).
Acknowledgements
This work was supported in part by the National Cancer Institute (P01-CA080124, R35-CA197743, U01-CA224173, and R01-CA208205 to R.K.J.; F32-CA216944 to H.T.N), the American Association of Cancer Research (19-40-50-DATT to M.D.), the Susan G. Komen Foundation (PDF14201739 to G.S.), and the European Research Council (ERC; 805225 to G.S.). R.K.J.’s research is also supported by grants from the National Foundation for Cancer Research, Harvard Ludwig Center, Jane’s Trust Foundation, and the Bill and Melinda Gates Foundation.
Author information
Authors and Affiliations
Contributions
H.T.N., M.D., G.S., W.W.H., S.R., P.H., L.L.M., and R.K.J. designed the study; H.T.N., M.D., G.S., S.R., and P.H. acquired the data. H.T.N., M.D., G.S., and R.K.J. contributed to analysis and interpretation of the data. H.T.N., M.D., G.S., S.Z., W.W.H., S.R., P.H., L.L.M., and R.K.J. were involved in drafting the article and revising it for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
R.K.J. received an honorarium from Amgen; consultant fees from Chugai, Enlight, Merck, Ophthotech, Pfizer, SPARC, SynDevRx, and XTuit; owns equity in Enlight, Ophthotech, and SynDevRx; and serves on the boards of trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. No funding or reagents from these companies were used in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Seano, G. et al. Nat. Biomed. Eng. 3, 230–245 (2019): https://doi.org/10.1038/s41551-018-0334-7
Supplementary information
Rights and permissions
About this article
Cite this article
Nia, H.T., Datta, M., Seano, G. et al. In vivo compression and imaging in mouse brain to measure the effects of solid stress. Nat Protoc 15, 2321–2340 (2020). https://doi.org/10.1038/s41596-020-0328-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0328-2
This article is cited by
-
Crystal ribcage: a platform for probing real-time lung function at cellular resolution
Nature Methods (2023)
-
Intravital measurements of solid stresses in tumours reveal length-scale and microenvironmentally dependent force transmission
Nature Biomedical Engineering (2023)
-
Biophysics in tumor growth and progression: from single mechano-sensitive molecules to mechanomedicine
Oncogene (2023)
-
A biomechanical view of epigenetic tumor regulation
Journal of Biological Physics (2023)
-
Cyclosporine A loaded brain targeting nanoparticle to treat cerebral ischemia/reperfusion injury in mice
Journal of Nanobiotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.