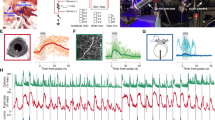
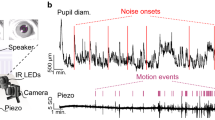
Abstract
The locus coeruleus (LC) is a region in the brainstem that produces noradrenaline and is involved in both normal and pathological brain function. Pupillometry, the measurement of pupil diameter, provides a powerful readout of LC activity in rodents, primates and humans. The protocol detailed here describes a miniaturized setup that can screen LC activity in rodents in real-time and can be established within 1–2 d. Using low-cost Raspberry Pi computers and cameras, the complete custom-built system costs only ~300 euros, is compatible with stereotaxic surgery frames and seamlessly integrates into complex experimental setups. Tools for pupil tracking and a user-friendly Pupillometry App allow quantification, analysis and visualization of pupil size. Pupillometry can discriminate between different, physiologically relevant firing patterns of the LC and can accurately report LC activation as measured by noradrenaline turnover. Pupillometry provides a rapid, non-invasive readout that can be used to verify accurate placement of electrodes/fibers in vivo, thus allowing decisions about the inclusion/exclusion of individual animals before experiments begin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during the current study (‘Anticipated results’) are available from the corresponding authors upon request.
Code availability
All software and code described in this protocol are freely available online:
Raspberry Pi Code https://github.com/ein-lab/pupillometry-raspi
MATLAB Code https://github.com/ein-lab/pupillometry-matlab
Pupillometry App
https://bohaceklab.hest.ethz.ch/pupillometry/ (web version)
https://github.com/ETHZ-INS/pupillometry (source code)
Change history
14 January 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41596-021-00493-6
References
Sara, S. J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10, 211–223 (2009).
Berridge, C. W. & Waterhouse, B. D. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 42, 33–84 (2003).
Chandler, D. J. et al. Redefining noradrenergic neuromodulation of behavior: impacts of a modular locus coeruleus architecture. J. Neurosci. 39, 8239–8249 (2019).
McCall, J. G. et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015).
Naegeli, C. et al. Locus coeruleus activity mediates hyperresponsiveness in posttraumatic stress disorder. Biol. Psychiatry 83, 254–262 (2018).
Isingrini, E. et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat. Neurosci. 19, 560–563 (2016).
Fortress, A. M. et al. Designer receptors enhance memory in a mouse model of Down syndrome. J. Neurosci. 35, 1343–1353 (2015).
Vermeiren, Y. & De Deyn, P. P. Targeting the norepinephrinergic system in Parkinson’s disease and related disorders: the locus coeruleus story. Neurochem. Int. 102, 22–32 (2017).
Delaville, C., Deurwaerdère, P. D. & Benazzouz, A. Noradrenaline and Parkinson’s disease. Front. Syst. Neurosci. 5, 1–12 (2011).
Mather, M. & Harley, C. W. The locus coeruleus: essential for maintaining cognitive function and the aging brain. Trends Cogn. Sci. 20, 214–226 (2016).
Peterson, A. C. & Li, C. S. R. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s diseases—an overview of imaging studies. Front. Aging Neurosci. 10, 1–16 (2018).
Weinshenker, D. Long road to ruin: noradrenergic dysfunction in neurodegenerative disease. Trends Neurosci. 41, 211–223 (2018).
Sara, S. J. & Bouret, S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76, 130–141 (2012).
Manaye, K. F., McIntire, D. D., Mann, D. M. A. & German, D. C. Locus coeruleus cell loss in the aging human brain: a non‐random process. J. Comp. Neurol. 358, 79–87 (1995).
Murphy, P. R., O’Connell, R. G., O’Sullivan, M., Robertson, I. H. & Balsters, J. H. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 35, 4140–4154 (2014).
Joshi, S., Li, Y., Kalwani, R. M. & Gold, J. I. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221–234 (2016).
Wang, C.-A., Blohm, G., Huang, J., Boehnke, S. E. & Munoz, D. P. Multisensory integration in orienting behavior: pupil size, microsaccades, and saccades. Biol. Psychol. 129, 36–44 (2017).
Lehmann, S. J. & Corneil, B. D. Transient pupil dilation after subsaccadic microstimulation of primate frontal eye fields. J. Neurosci. 36, 3765–3776 (2016).
Varazzani, C., San-Galli, A., Gilardeau, S. & Bouret, S. Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. J. Neurosci. 35, 7866–7877 (2015).
Schwalm, M. & Jubal, E. R. Back to pupillometry: how cortical network state fluctuations tracked by pupil dynamics could explain neural signal variability in human cognitive neuroscience. eNeuro 4, ENEURO.0293-16.2017 (2017).
Reimer, J. et al. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362 (2014).
Reimer, J. et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 7, 13289 (2016).
Breton-Provencher, V. & Sur, M. Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci. 22, 218–228 (2019).
Zerbi, V. et al. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718.e5 (2019).
Zuend, M. et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat. Metab. 2, 179–191 (2020).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Nath, T. et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152–2176 (2019).
Uematsu, A. et al. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 20, 1602–1611 (2017).
Schwarz, L. A. & Luo, L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 25, R1051–R1056 (2015).
Liu, Y., Rodenkirch, C., Moskowitz, N., Schriver, B. & Wang, Q. Dynamic lateralization of pupil dilation evoked by locus coeruleus activation results from sympathetic, not parasympathetic, contributions. Cell Rep. 20, 3099–3112 (2017).
Patriarchi, T. et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 4422, eaat4422 (2018).
Feng, J. et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 102, 745–761.e8 (2019).
Patriarchi, T. et al. Imaging neuromodulators with high spatiotemporal resolution using genetically encoded indicators. Nat. Protoc. 14, 3471–3505 (2019).
de Gee, J. W. et al. Dynamic modulation of decision biases by brainstem arousal systems. eLife 6, e23232 (2017).
Grueschow, M., Polania, R., Hare, T. A. & Ruff, C. C. Arousal optimizes neural evidence representation for human decision-making. Preprint at Cell Press https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3155606.
Hellrung, L. et al. Flexible adaptive paradigms for fMRI using a novel software package ‘Brain Analysis in Real-Time’ (BART). PLoS One 10, e0118890 (2015).
Meyer, A. F., Poort, J., O’Keefe, J., Sahani, M. & Linden, J. F. A head-mounted camera system integrates detailed behavioral monitoring with multichannel electrophysiology in freely moving mice. Neuron 100, 46–60.e7 (2018).
Parlato, R., Otto, C., Begus, Y., Stotz, S. & Schutz, G. Specific ablation of the transcription factor CREB in sympathetic neurons surprisingly protects against developmentally regulated apoptosis. Development 134, 1663–1670 (2007).
Gilzenrat, M. S., Nieuwenhuis, S., Jepma, M. & Cohen, J. D. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn. Affect. Behav. Neurosci. 10, 252–269 (2010).
Vazey, E. M. & Aston-Jones, G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl Acad. Sci. USA 111, 3859–3864 (2014).
Gomez, J. L. et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Aston-Jones, G. & Bloom, F. E. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J. Neurosci. 1, 887–900 (1981).
Foote, S. L., Aston-Jones, G. & Bloom, F. E. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl Acad. Sci. USA 77, 3033–3037 (1980).
Clayton, E. C., Rajkowski, J., Cohen, J. D. & Aston-Jones, G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J. Neurosci. 24, 9914–9920 (2004).
Aston-Jones, G. & Cohen, J. D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
Aston-Jones, G., Foote, S. L. & Bloom, F. E. Low doses of ethanol disrupt sensory responses of brain noradrenergic neurones. Nature 296, 857–860 (1982).
Chen, F.-J. & Sara, S. J. Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience 144, 472–481 (2007).
Van Dam, D., Vermeiren, Y., Aerts, T. & De Deyn, P. P. Novel and sensitive reversed-phase high-pressure liquid chromatography method with electrochemical detection for the simultaneous and fast determination of eight biogenic amines and metabolites in human brain tissue. J. Chromatogr. A 1353, 28–39 (2014).
Vermeiren, Y. et al. The monoaminergic footprint of depression and psychosis in dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res. Ther. 7, 7 (2015).
Janssens, J. et al. Cerebrospinal fluid and serum MHPG improve Alzheimer’s disease versus dementia with Lewy bodies differential diagnosis. Alzheimers Dement. 10, 172–181 (2018).
Vogt, N. Optogenetics turns up the heat. Nat. Methods 16, 681 (2019).
Owen, S. F., Liu, M. H. & Kreitzer, A. C. Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci. 22, 1061–1065 (2019).
Davson, H. Physiology of the Eye 4th edn, 468–477 (Academic Press: 1980).
Acknowledgements
B.W. acknowledges support by the University of Zurich and the Swiss National Science Foundation (grant 310030_182703). J.B. acknowledges support by the ETH Zurich, ETH Project Grant ETH-20 19-1, and the Swiss National Science Foundation (grant 310030_172889/1). The authors acknowledge Rongrong Xiang and Matthew J. P. Barrett for their initial work on the MATLAB analysis, Marc Zuend for extensive testing of prototypes, Christa Schläppi for testing the pupillometry guidelines and Alexandra von Faber-Castell for testing the assembly guide.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.P., K.D.F., B.W. and J.B.; methodology, M.P., K.D.F. and O.S.; investigation, M.P., K.D.F, A.F.-S., S.N.D., Y.V. and M.T.W.; software, K.D.F., L.M.v.Z., P.-L.G. and O.S.; writing—original draft, M.P., K.D.F, L.M.v.Z., O.S., S.N.D. and J.B.; figures, M.P. and K.D.F.; writing—review and editing, M.P., K.D.F., L.M.v.Z., O.S., A.F.-S., P.-L.G., Y.V., S.N.D., M.T.W., P.P.D.D., B.W. and J.B.; funding acquisition, B.W., P.P.D.D. and J.B.; resources, B.W. and J.B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Zerbi, V. et al. Neuron 103, 702–718.e5 (2019): https://doi.org/10.1016/j.neuron.2019.05.034
Zuend, M. et al. Nat. Metab. 2, 179–191 (2020): https://doi.org/10.1038/s42255-020-0170-4
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1 and 2 and Supplementary Manual.
Rights and permissions
About this article
Cite this article
Privitera, M., Ferrari, K.D., von Ziegler, L.M. et al. A complete pupillometry toolbox for real-time monitoring of locus coeruleus activity in rodents. Nat Protoc 15, 2301–2320 (2020). https://doi.org/10.1038/s41596-020-0324-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0324-6
This article is cited by
-
Centripetal integration of past events in hippocampal astrocytes regulated by locus coeruleus
Nature Neuroscience (2024)
-
Control and coding of pupil size by hypothalamic orexin neurons
Nature Neuroscience (2023)
-
The integrity of dopaminergic and noradrenergic brain regions is associated with different aspects of late-life memory performance
Nature Aging (2023)
-
Pupil-linked arousal with very light exercise: pattern of pupil dilation during graded exercise
The Journal of Physiological Sciences (2022)
-
An Easily Compatible Eye-tracking System for Freely-moving Small Animals
Neuroscience Bulletin (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.