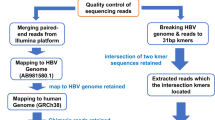
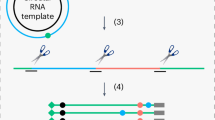
Abstract
Circular DNA is ubiquitous in nature in the form of plasmids, circular DNA viruses, and extrachromosomal circular DNA (eccDNA) in eukaryotes. Sequencing of such molecules is essential to profiling virus distributions, discovering new viruses and understanding the roles of eccDNAs in eukaryotic cells. Circular DNA enrichment sequencing (CIDER-Seq) is a technique to enrich and accurately sequence circular DNA without the need for polymerase chain reaction amplification, cloning, and computational sequence assembly. The approach is based on randomly primed circular DNA amplification, which is followed by several enzymatic DNA repair steps and then by long-read sequencing. CIDER-Seq includes a custom data analysis package (CIDER-Seq Data Analysis Software 2) that implements the DeConcat algorithm to deconcatenate the long sequencing products of random circular DNA amplification into the intact sequences of the input circular DNA. The CIDER-Seq data analysis package can generate full-length annotated virus genomes, as well as circular DNA sequences of novel viruses. Applications of CIDER-Seq also include profiling of eccDNA molecules such as transposable elements (TEs) from biological samples. The method takes ~2 weeks to complete, depending on the computational resources available. Owing to the present constraints of long-read single-molecule sequencing, the accuracy of circular virus and eccDNA sequences generated by the CIDER-Seq method scales with sequence length, and the greatest accuracy is obtained for molecules <10 kb long.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Raw and processed data from the original publication are freely available at https://doi.org/10.5281/zenodo.1009036. Data from Fig. 1 are available upon request. Fig. 3 has associated raw data that are provided in the software installation.
Code availability
The CIDER-Seq Data Analysis Software package is available under a GNU Affero Public License v. 3.0 at https://github.com/devang-mehta/ciderseq2. A static copy of the software version used in developing this protocol has been deposited at https://doi.org/10.5281/zenodo.3386568. A Singularity image of the software is also available at https://doi.org/10.5281/zenodo.3401571.
Change history
27 May 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41596-022-00711-9
References
De Sales Lima, F. E. et al. Genomic characterization of novel circular ssDNA viruses from insectivorous bats in Southern Brazil. PLoS One 10, 1–11 (2015).
Hansen, T. A. et al. New type of papillomavirus and novel circular single stranded DNA virus discovered in urban Rattus norvegicus using circular DNA enrichment and metagenomics. PLoS One 10, 1–11 (2015).
Jelen, M. M. et al. Global genomic diversity of human papillomavirus 6 based on 724 isolates and 190 complete genome sequences. J. Virol. 88, 7307–7316 (2014).
Mehta, D. et al. A new full-length circular DNA sequencing method for viral-sized genomes reveals that RNAi transgenic plants provoke a shift in geminivirus populations in the field. Nucleic Acids Res. 47, e9 (2019).
Rey, C. & Vanderschuren, H. V. Cassava mosaic and brown streak diseases: current perspectives and beyond. Annu. Rev. Virol. 4, 429–452 (2017).
Shibata, Y. et al. Extrachromosomal microDNAs and chromosomal microdeletions in normal tissues. Science 336, 82–86 (2012).
Møller, H. D. et al. Circular DNA elements of chromosomal origin are common in healthy human somatic tissue. Nat. Commun. 9, 1–12 (2018).
Kumar, P. et al. Normal and cancerous tissues release extrachromosomal circular DNA (eccDNA) into the circulation. Mol. Cancer Res. 15, 1–8 (2017).
Koo, D.-H. et al. Extrachromosomal circular DNA-based amplification and transmission of herbicide resistance in crop weed Amaranthus palmeri. Proc. Natl Acad. Sci. USA 115, 3332–3337 (2018).
Zhang, K. et al. Sequencing genomes from single cells by polymerase cloning. Nat. Biotechnol. 24, 680–686 (2006).
Whon, T. W. et al. Metagenomic characterization of airborne viral DNA diversity in the near-surface atmosphere. J. Virol. 86, 8221–8231 (2012).
Rosario, K., Morrison, C. M., Mettel, K. A. & Betancourt, W. Q. Novel circular Rep-encoding single-stranded DNA viruses detected in treated wastewater. Microbiol. Resour. Announc. 8, 9–10 (2019).
Møller, H. D., Parsons, L., Jørgensen, T. S., Botstein, D. & Regenberg, B. Extrachromosomal circular DNA is common in yeast. Proc. Natl Acad. Sci. USA 112, E3114–E3122 (2015).
Shoura, M. J. et al. Intricate and cell type-specific populations of endogenous circular DNA (eccDNA) in Caenorhabditis elegans and Homo sapiens. G3 (Bethesda) 7, 3295–3303 (2017).
Lanciano, S. et al. Sequencing the extrachromosomal circular mobilome reveals retrotransposon activity in plants. PLoS Genet. 13, e1006630 (2017).
Møller, H. D. et al. Formation of extrachromosomal circular DNA from long terminal repeats of retrotransposons in Saccharomyces cerevisiae. G3 (Bethesda) 6, 453–462 (2016).
Volden, R. et al. Improving nanopore read accuracy with the R2C2 method enables the sequencing of highly multiplexed full-length single-cell cDNA. Proc. Natl Acad. Sci. USA 115, 9726–9731 (2018).
Roux, S. et al. Minimum information about an uncultivated virus genome (MIUVIG). Nat. Biotechnol. 37, 29–37 (2019).
Nooij, S., Schmitz, D., Vennema, H., Kroneman, A. & Koopmans, M. P. G. Overview of virus metagenomic classification methods and their biological applications. Front. Microbiol. 9, 749 (2018).
Fu, L., Niu, B., Zhu, Z., Wu, S. & Li, W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152 (2012).
Li, W. & Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006).
Chang, S., Puryear, J. & Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Report. 11, 113–116 (1993).
Pacific Biosciences. SMRT® Analysis Software Installation (v.2.3.0). https://smrt-analysis.readthedocs.io/en/latest/SMRT-Analysis-Software-Installation-v2.3.0/
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797 (2004).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinformatics 10, 421 (2009).
Cock, P. J. A. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Pacific Biosciences. Procedure & Checklist - Preparing MagBeads for Sequencing (2015): https://s3.amazonaws.com/files.pacb.com/pdf/Procedure_Checklist_Preparing_MagBeads_for-Sequencing.pdf
Pacific Biosciences. SMRT Link User Guide. Version 06 (2018): https://www.pacb.com/wp-content/uploads/SMRT_Link_User_Guide_v600.pdf
Kurtzer, G. M., Sochat, V. & Bauer, M. W. Singularity: scientific containers for mobility of compute. PLoS One 12, 1–20 (2017).
Acknowledgements
We thank A. Patrignani (Functional Genomics Center Zurich, Switzerland) for assistance with SMRT sequencing, as well as for helpful advice and discussion. The protocol for geminivirus sequencing was initially developed during a project funded by the European Union’s Seventh Framework Programme for research, technological development, and demonstration (EUGA–2013–608422-IDP BRIDGES to H.V. & D.M.). The authors acknowledge financial support from the Belgian FNRS (grant M.i.S. F.4515.17 to H.V.; grant 1.B456.20 to S.S.Z. and H.V.), LEAPAgri grant 288 to H.V., and a Swiss National Science Foundation Early Postdoc Mobility grant (181602) to D.M.
Author information
Authors and Affiliations
Contributions
Conceptualization: D.M. and H.V.; methodology and formal analysis: D.M., M.H.-H., L.C., S.S.Z.; software: M.H.-H., L.C. and D.M.; visualization and writing: D.M.; resources and supervision: H.V. All authors agreed with the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Simon Roux and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Mehta, D. et al. Nucleic Acids Res. 47, e9 (2019): https://doi.org/10.1093/nar/gky914
Integrated supplementary information
Supplementary Fig. 1 Example profiles of enriched circular DNA.
a. Cassava DNA samples after CIDER-Seq enrichment run on a Bioanalyzer 2100 High-sensitivity DNA chip. b. Arabidopsis DNA samples after CIDER-Seq enrichment run on a Tapestation Genomic DNA Screentape.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Rights and permissions
About this article
Cite this article
Mehta, D., Cornet, L., Hirsch-Hoffmann, M. et al. Full-length sequencing of circular DNA viruses and extrachromosomal circular DNA using CIDER-Seq. Nat Protoc 15, 1673–1689 (2020). https://doi.org/10.1038/s41596-020-0301-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0301-0
This article is cited by
-
TeCD: The eccDNA Collection Database for extrachromosomal circular DNA
BMC Genomics (2023)
-
Microevolutionary dynamics of eccDNA in Chinese hamster ovary cells grown in fed-batch cultures under control and lactate-stressed conditions
Scientific Reports (2023)
-
Extrachromosomal circular DNA and structural variants highlight genome instability in Arabidopsis epigenetic mutants
Nature Communications (2023)
-
Genome-wide characterization of extrachromosomal circular DNA in gastric cancer and its potential role in carcinogenesis and cancer progression
Cellular and Molecular Life Sciences (2023)
-
ECCsplorer: a pipeline to detect extrachromosomal circular DNA (eccDNA) from next-generation sequencing data
BMC Bioinformatics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.