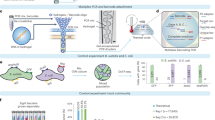
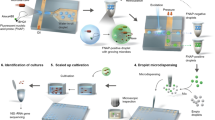
Abstract
Stable isotope labeling of microbial taxa of interest and their sorting provide an efficient and direct way to answer the question “who does what?” in complex microbial communities when coupled with fluorescence in situ hybridization or downstream ‘omics’ analyses. We have developed a platform for automated Raman-based sorting in which optical tweezers and microfluidics are used to sort individual cells of interest from microbial communities on the basis of their Raman spectra. This sorting of cells and their downstream DNA analysis, such as by mini-metagenomics or single-cell genomics, or cultivation permits a direct link to be made between the metabolic roles and the genomes of microbial cells within complex microbial communities, as well as targeted isolation of novel microbes with a specific physiology of interest. We describe a protocol from sample preparation through Raman-activated live cell sorting. Subsequent cultivation of sorted cells is described, whereas downstream DNA analysis involves well-established approaches with abundant methods available in the literature. Compared with manual sorting, this technique provides a substantially higher throughput (up to 500 cells per h). Furthermore, the platform has very high sorting accuracy (98.3 ± 1.7%) and is fully automated, thus avoiding user biases that might accompany manual sorting. We anticipate that this protocol will empower in particular environmental and host-associated microbiome research with a versatile tool to elucidate the metabolic contributions of microbial taxa within their complex communities. After a 1-d preparation of cells, sorting takes on the order of 4 h, depending on the number of cells required.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Code availability
The MATLAB code to operate the RACS is included in Supplementary Software 1 and 2 of this paper and is also available from GitHub (https://github.com/harubang2/MATLAB-platform-for-Raman-activated-cell-sorting-RACS).
References
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019).
Blainey, P. C., Mosier, A. C., Potanina, A., Francis, C. A. & Quake, S. R. Genome of a low-salinity ammonia-oxidizing archaeon determined by single-cell and metagenomic analysis. PLoS ONE 6, e16626 (2011).
Thomas, T., Gilbert, J. & Meyer, F. Metagenomics -– a guide from sampling to data analysis. Microb. Inform. Exp. 2, 3 (2012).
Horgan, R. P. & Kenny, L. C. ‘Omic’ technologies: genomics, transcriptomics, proteomics and metabolomics. Obstet. Gynaecol 13, 189–195 (2011).
Prosser, J. I. Dispersing misconceptions and identifying opportunities for the use of ‘omics’ in soil microbial ecology. Nat. Rev. Microbiol. 13, 439–446 (2015).
Yu, F. B. et al. Microfluidic-based mini-metagenomics enables discovery of novel microbial lineages from complex environmental samples. eLife 6, e26580 (2017).
Mukherjee, S. et al. Genomes OnLine database (GOLD) v.7: updates and new features. Nucleic Acids Res 47, D649–D659 (2019).
Woyke, T., Doud, D. F. R. & Schulz, F. The trajectory of microbial single-cell sequencing. Nat. Methods 14, 1045–1054 (2017).
Berry, D. & Loy, A. Stable-isotope probing of human and animal microbiome function. Trends Microbiol 26, 999–1007 (2018).
Manefield, M., Whiteley, A. S., Griffiths, R. I. & Bailey, M. J. RNA stable isotope probing, a novel means of linking microbial community function to phylogeny. Appl. Environ. Microbiol. 68, 5367–5373 (2002).
Dumont, M. G. & Murrell, J. C. Stable isotope probing—linking microbial identity to function. Nat. Rev. Microbiol. 3, 499–504 (2005).
Wilhelm, R. C., Singh, R., Eltis, L. D. & Mohn, W. W. Bacterial contributions to delignification and lignocellulose degradation in forest soils with metagenomic and quantitative stable isotope probing. ISME J 13, 413–429 (2019).
Wang, Y., Huang, W. E., Cui, L. & Wagner, M. Single cell stable isotope probing in microbiology using Raman microspectroscopy. Curr. Opin. Biotechnol. 41, 34–42 (2016).
Haider, S. et al. Raman microspectroscopy reveals long-term extracellular activity of chlamydiae. Mol. Microbiol 77, 687–700 (2010).
Huang, W. E. et al. Raman-FISH: combining stable-isotope Raman spectroscopy and fluorescence in situ hybridization for the single cell analysis of identity and function. Environ. Microbiol. 9, 1878–1889 (2007).
Wagner, M. Single-cell ecophysiology of microbes as revealed by Raman microspectroscopy or secondary ion mass spectrometry imaging. Annu. Rev. Microbiol. 63, 411–429 (2009).
Berry, D. et al. Tracking heavy water (D2O) incorporation for identifying and sorting active microbial cells. Proc. Natl Acad. Sci. USA 112, E194–E203 (2015).
Malmstrom, R. R. & Eloe-Fadrosh, E. A. Advancing genome-resolved metagenomics beyond the shotgun. mSystems 4, e00118–e00119 (2019).
Neufeld, J. D. et al. DNA stable-isotope probing. Nat. Protoc. 2, 860–866 (2007).
Jing, X. et al. Raman-activated cell sorting and metagenomic sequencing revealing carbon-fixing bacteria in the ocean. Environ. Microbiol. 20, 2241–2255 (2018).
Wang, Y. et al. Raman activated cell ejection for isolation of single cells. Anal. Chem. 85, 10697–10701 (2013).
Singer, E., Wagner, M. & Woyke, T. Capturing the genetic makeup of the active microbiome in situ. ISME J 11, 1949–1963 (2017).
Huang, W. E., Ward, A. D. & Whiteley, A. S. Raman tweezers sorting of single microbial cells. Environ. Microbiol. Rep 1, 44–49 (2009).
Lee, K. S. et al. An automated Raman-based platform for the sorting of live cells by functional properties. Nat. Microbiol. 4, 1035–1048 (2019).
Lee, K. S., Wagner, M. & Stocker, R. Raman-based sorting of microbial cells to link functions to their genes. Microb. Cell 7, 62–65 (2020).
Premvardhan, L., Bordes, L., Beer, A., Büchel, C. & Robert, B. Carotenoid structures and environments in trimeric and oligomeric fucoxanthin chlorophyll a/c2 proteins from resonance Raman spectroscopy. J. Phys. Chem. B 113, 12565–12574 (2009).
Takano, H. The regulatory mechanism underlying light-inducible production of carotenoids in nonphototrophic bacteria. Biosci. Biotechnol. Biochem. 80, 1264–1273 (2016).
Wagstaff, K., Cardie, C., Rogers, S. & Schrödl, S. Constrained k-means clustering with background knowledge. in Proc. 18th International Conference on Machine Learning (eds Brodley, C. E. & Danyluk, A. P.) 577–584 (Morgan Kaufmann, 2001).
Kanungo, T. et al. An efficient k-means clustering algorithm: analysis and implementation. IEEE Trans. Patt. Anal. Mach. Intell. 24, 881–892 (2002).
Rinke, C. et al. Obtaining genomes from uncultivated environmental microorganisms using FACS-based single-cell genomics. Nat. Protoc. 9, 1038–1048 (2014).
Bonner, W. A., Hulett, H. R., Sweet, R. G. & Herzenberg, L. A. Fluorescence activated cell sorting. Rev. Sci. Instrum. 43, 404–409 (1972).
Ha, B. H., Lee, K. S., Jung, J. H. & Sung, H. J. Three-dimensional hydrodynamic flow and particle focusing using four vortices Dean flow. Microfluid. Nanofluid. 17, 647–655 (2014).
Chu, H., Doh, I. & Cho, Y.-H. A three-dimensional (3D) particle focusing channel using the positive dielectrophoresis (pDEP) guided by a dielectric structure between two planar electrodes. Lab Chip 9, 686–691 (2009).
Gao, C. et al. Single-cell bacterial transcription measurements reveal the importance of dimethylsulfoniopropionate (DMSP) hotspots in ocean sulfur cycling. Nat. Commun. 11, 1942 (2020).
Kitzinger, K. et al. Single cell analyses reveal contrasting life strategies of the two main nitrifiers in the ocean. Nat. Commun. 11, 767 (2020).
Majed, N., Chernenko, T., Diem, M. & Gu, A. Z. Identification of functionally relevant populations in enhanced biological phosphorus removal processes based on intracellular polymers profiles and insights into the metabolic diversity and heterogeneity. Environ. Sci. Technol. 46, 5010–5017 (2012).
Fernando, E. Y. et al. Resolving the individual contribution of key microbial populations to enhanced biological phosphorus removal with Raman–FISH. ISME J 13, 1933–1946 (2019).
Milucka, J. et al. Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 491, 541–546 (2012).
Hatzenpichler, R. et al. Visualizing in situ translational activity for identifying and sorting slow-growing archaeal–bacterial consortia. Proc. Natl Acad. Sci. USA 113, E4069–E4078 (2016).
Schiessl, K. T. et al. Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat. Commun. 10, 762 (2019).
Gleizer, S. et al. Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 179, 1255–1263 (2019).
Dong, T. G., Ho, B. T., Yoder-Himes, D. R. & Mekalanos, J. J. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc. Natl Acad. Sci. USA 110, 2623–2628 (2013).
Dolinšek, J., Lagkouvardos, I., Wanek, W., Wagner, M. & Daims, H. Interactions of nitrifying bacteria and heterotrophs: identification of a Micavibrio-like putative predator of Nitrospira spp. Appl. Environ. Microbiol. 79, 2027–2037 (2013).
Pätzold, R. et al. In situ mapping of nitrifiers and anammox bacteria in microbial aggregates by means of confocal resonance Raman microscopy. J. Microbiol. Methods 72, 241–248 (2008).
Wei, L. & Min, W. Electronic preresonance stimulated Raman scattering microscopy. J. Phys. Chem. Lett. 9, 4294–4301 (2018).
Gruber-Vodicka, H. R. et al. Paracatenula, an ancient symbiosis between thiotrophic Alphaproteobacteria and catenulid flatworms. Proc. Natl Acad. Sci. USA. 108, 12078–12083 (2011).
Lenz, R., Enders, K., Stedmon, C. A., MacKenzie, D. M. A. & Nielsen, T. G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 100, 82–91 (2015).
Gillibert, R. et al. Raman tweezers for small microplastics and nanoplastics identification in seawater. Environ. Sci. Technol. 53, 9003–9013 (2019).
Choy, C. A. et al. The vertical distribution and biological transport of marine microplastics across the epipelagic and mesopelagic water column. Sci. Rep. 9, 7843 (2019).
Zhang, P. et al. Raman-activated cell sorting based on dielectrophoretic single-cell trap and release. Anal. Chem. 87, 2282–2289 (2015).
McIlvenna, D. et al. Continuous cell sorting in a flow based on single cell resonance Raman spectra. Lab Chip 16, 1420–1429 (2016).
Folick, A., Min, W. & Wang, M. C. Label-free imaging of lipid dynamics using coherent anti-stokes Raman scattering (CARS) and stimulated Raman scattering (SRS) microscopy. Curr. Opin. Genet. Dev. 21, 585–590 (2011).
Hiramatsu, K. et al. High-throughput label-free molecular fingerprinting flow cytometry. Sci. Adv. 5, eaau0241 (2019).
Suzuki, Y. et al. Label-free chemical imaging flow cytometry by high-speed multicolor stimulated Raman scattering. Proc. Natl Acad. Sci. USA 116, 15842–15848 (2019).
Nitta, N. et al. Raman image-activated cell sorting. Nat. Commun. 11, 3452 (2020).
Eek, K. M., Sessions, A. L. & Lies, D. P. Carbon-isotopic analysis of microbial cells sorted by flow cytometry. Geobiology 5, 85–95 (2007).
Dyksma, S. et al. Ubiquitous Gammaproteobacteria dominate dark carbon fixation in coastal sediments. ISME J 10, 1939–1953 (2016).
Ling, L., Zhou, F., Huang, L. & Li, Z.-Y. Optical forces on arbitrary shaped particles in optical tweezers. J. Appl. Phys. 108, 073110 (2010).
Bonessi, D., Bonin, K. & Walker, T. Optical forces on particles of arbitrary shape and size. J. Opt. A Pure Appl. Opt. 9, S228–S234 (2007).
Ashkin, A. Forces of a single-beam gradient laser trap on a dielectric sphere in the ray optics regime. Biophys. J. 61, 569–582 (1992).
Novotny, L., Bian, R. X. & Xie, X. S. Theory of nanometric optical tweezers. Phys. Rev. Lett. 79, 645–648 (1997).
Dholakia, K. & Reece, P. Optical micromanipulation takes hold. Nano Today 1, 18–27 (2006).
Kim, S., Kang, I., Seo, J.-H. & Cho, J.-C. Culturing the ubiquitous freshwater actinobacterial acI lineage by supplying a biochemical ‘helper’ catalase. ISME J 13, 2252–2263 (2019).
Li, T. et al. Simultaneous analysis of microbial identity and function using NanoSIMS. Environ. Microbiol. 10, 580–588 (2008).
Huang, W. E., Griffiths, R. I., Thompson, I. P., Bailey, M. J. & Whiteley, A. S. Raman microscopic analysis of single microbial cells. Anal. Chem. 76, 4452–4458 (2004).
McDonald, J. C. et al. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21, 27–40 (2000).
Schuster, K. C., Reese, I., Urlaub, E., Gapes, J. R. & Lendl, B. Multidimensional information on the chemical composition of single bacterial cells by confocal Raman microspectroscopy. Anal. Chem. 72, 5529–5534 (2000).
Dochow, S. et al. Quartz microfluidic chip for tumour cell identification by Raman spectroscopy in combination with optical traps. Anal. Bioanal. Chem. 405, 2743–2746 (2013).
Kodinariya, T. M. & Makwana, P. R. Review on determining number of Cluster in K-Means Clustering. Int. J. Adv. Res. Comput. Sci. Manag. Stud. 1, 90–95 (2013).
Bjerg, J. T. et al. Long-distance electron transport in individual, living cable bacteria. Proc. Natl Acad. Sci. USA. 115, 5786–5791 (2018).
Zhao, J., Lui, H., McLean, D. I. & Zeng, H. Automated autofluorescence background subtraction algorithm for biomedical Raman spectroscopy. Appl. Spectrosc. 61, 1225–1232 (2007).
Beier, B. D. & Berger, A. J. Method for automated background subtraction from Raman spectra containing known contaminants. Analyst 134, 1198–1202 (2009).
Hehemann, J.-H. et al. Adaptive radiation by waves of gene transfer leads to fine-scale resource partitioning in marine microbes. Nat. Commun. 7, 12860 (2016).
Taheri-Araghi, S. et al. Cell-size control and homeostasis in bacteria. Curr. Biol. 25, 385–391 (2015).
Mazutis, L. et al. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 8, 870–891 (2013).
Wang, Y. et al. Reverse and multiple stable isotope probing to study bacterial metabolism and interactions at the single cell level. Anal. Chem. 88, 9443–9450 (2016).
Yuan, X. et al. Effect of laser irradiation on cell function and its implications in Raman spectroscopy. Appl. Environ. Microbiol. 84, e02508–e02517 (2018).
Acknowledgements
We acknowledge support from a US Department of Energy Joint Genome Institute Emerging Technologies Opportunity grant (DE-AC02-05CH11231 to R.S. and M.W.). R.S. acknowledges support from a Gordon and Betty Moore Foundation Marine Microbial 1775 Initiative Investigator Award (GBMF3783), a Gordon and Betty Moore Symbiosis in Aquatic Systems Initiative Investigator Award 1776 (GBMF9197), a grant from the Simons Foundation (542395) as part of the Principles of Microbial Ecosystems (PriME) Collaborative and a grant (#315230_176189) from the Swiss National Science Foundation. F.C.P. and D.B. were supported by the Austrian Science Fund (FWF; P26127-B20 and P27831-B28) and the European Research Council (Starting Grant: FunKeyGut 741623). F.C.P. was also supported by the European Union’s Horizon 2020 Framework Programme for Research and Innovation (grant no.658718). M.P. and M.W. were supported by the European Research Council via the Advanced Grant project NITRICARE 294343 and the FWF Wittgenstein award (to M.W.). L.B. was supported by grants from the Swedish Research Council (2019-04401) and the Science for Life Laboratory. We gratefully acknowledge funding from the European Molecular Biology Organization (EMBO; ALTF 1109-2016) and from the Human Frontier Science Program (HFSP; LT001209/2017) to U.A. We thank Horiba, Renishaw, and Bruker for providing their system specifications. We thank Cetoni for permission to display their software window. We thank R. Naisbit for scientific editing and M. Schmid for help with maintaining the Raman systems at the University of Vienna. We thank the M. Polz group, University of Vienna, Austria, for providing Vibrio alginolyticus 12G01 (wild type; NCBI:txid314288) and Vibrio cyclitrophicus 1F111 (wild type; NCBI:txid1136159). We thank S. Jun’s group, University of California, San Diego, for providing Escherichia coli NCM3722 ∆motA (non-motile mutant).
Author information
Authors and Affiliations
Contributions
K.S.L., F.C.P., M.P., L.B., U.A. and D.B. designed the protocol and performed the experiments. R.S. and M.W. supervised the research. K.S.L. and R.S. wrote the manuscript. All authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Wei Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Lee, K. S. et al. Nat. Microbiol. 4, 1035–1048 (2019): https://doi.org/10.1038/s41564-019-0394-9
Pereira, F. C. et al. Nat. Commun. 11, 5104 (2020): https://doi.org/10.1038/s41467-020-18928-1
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
41596_2020_427_MOESM3_ESM.mp4
Supplementary Video 1 Raman-activated cell sorting (RACS) procedures. The movie is composed of four sections: (i) visualization of flow in the microfluidic device; (ii) system calibration; (iii) case 1, the process for a captured and selected cell (sorted); and (iv) case 2, the process for a captured and rejected cell (not sorted). In section (i), the two separate flow streams do not interfere with each other–the sample flow traverses the analysis region and exits into the waste outlet by default, whereas the flow carries a cell released at the ‘release location’ (blue box) through the collection outlet. Section (ii) shows the Raman measurement of the working fluid (in the absence of a cell) to be used to calculate the PC values (eqn. (1) in the main text) during the RACS. For sections (iii) and (iv), the left and right panels represent the CCD image (obtained by the lower microscope) and the corresponding operation of the RACS software, respectively. The details of the sorting procedures are described in the lower panel of the movie.
41596_2020_427_MOESM4_ESM.zip
Supplementary Data 1 Computer-aided design (CAD) diagrams for the microfluidic sorter. The CAD file is provided as a separate supplement, “Supplementary_Data_1.dxf”. Users with access to a cleanroom facility can prepare the device from scratch (i.e., fabrication from a master mold using this photomask design and creation of the PDMS microfluidic device), whereas those who do not have expertise in microfabrication can ask commercial manufacturers to fabricate the master mold and then fabricate the PDMS microfluidic device in their laboratory.
Supplementary Data 2
Statistical source data for Supplementary Fig. 2
Supplementary Data 3
Statistical source data for Supplementary Fig. 3cd
41596_2020_427_MOESM7_ESM.zip
Supplementary Software 1 MATLAB GUI platform and corresponding script code to operate the RACS. ‘fig’ and ‘m’ files are bound together and thus should be identically named. See ‘README.pdf’ file that accompanies in the same zip file for system requirements and instructions to run this code.
41596_2020_427_MOESM8_ESM.zip
Supplementary Software 2 MATLAB GUI platform and corresponding script code to operate the RACS based on a machine-learning (K-means clustering) algorithm. ‘fig’ and ‘m’ files are bound together and thus should be identically named. See ‘README.pdf’ file that accompanies in the same zip file for system requirements and instructions to run this code.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5c
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
About this article
Cite this article
Lee, K.S., Pereira, F.C., Palatinszky, M. et al. Optofluidic Raman-activated cell sorting for targeted genome retrieval or cultivation of microbial cells with specific functions. Nat Protoc 16, 634–676 (2021). https://doi.org/10.1038/s41596-020-00427-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00427-8
This article is cited by
-
Long-read metagenomics paves the way toward a complete microbial tree of life
Nature Methods (2023)
-
Identification of inulin-responsive bacteria in the gut microbiota via multi-modal activity-based sorting
Nature Communications (2023)
-
Single-cell stable isotope probing in microbial ecology
ISME Communications (2022)
-
Microbial storage and its implications for soil ecology
The ISME Journal (2022)
-
Single-cell Raman-activated sorting and cultivation (scRACS-Culture) for assessing and mining in situ phosphate-solubilizing microbes from nature
ISME Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.