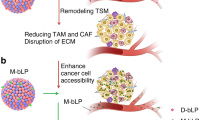
Abstract
Here we describe two protocols for the construction of responsive and activable nanomedicines that regulate the tumor microenvironment (TME). The TME is composed of all non-cellular and cellular components surrounding a tumor, including the surrounding blood vessels, immune cells, fibroblasts, signaling molecules, and extracellular matrix and has a crucial role in tumor initiation, growth, and metastasis. Owing to the relatively stable properties of the TME compared to tumor cells, which exhibit frequent genetic mutations and epigenetic changes, therapeutic strategies targeting the TME using multifunctional nanomedicines hold great potential for anti-tumor therapy. By regulating tumor-associated platelets and pancreatic stellate cells (PSCs), the two major players in the TME, we can effectively manipulate the physiological barriers for enhanced drug delivery and significantly improve the tumor penetration and therapeutic efficacy of chemotherapeutics. The preparation and characterization of the multifunctional nanoparticles takes ~10 h for tumor-associated platelet regulation and 16 h for PSC regulation. These nanoformulations can be readily applied to regulate other components in the TME to realize synergistic or additive anti-tumor activity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research papers https://doi.org/10.1038/s41551-017-0115-8 and https://doi.org/10.1038/s41467-018-05906-x. The raw datasets are too large to be publicly shared but are available for research purposes from the corresponding authors upon reasonable request.
References
Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8, 98–101 (1989).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Zhang, F. et al. Nanoparticles that reshape the tumor milieu create a therapeutic window for effective T-cell therapy in solid malignancies. Cancer Res. 78, 3718–3730 (2018).
Balkwill, F. R., Capasso, M. & Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 125, 5591–5596 (2012).
Ji, T. J., Zhao, Y., Ding, Y. P. & Nie, G. J. Using functional nanomaterials to target and regulate the tumor microenvironment: diagnostic and therapeutic applications. Adv. Mater. 25, 3508–3525 (2013).
Belli, C. et al. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 65, 22–32 (2018).
Yuan, Y., Jiang, Y. C., Sun, C. K. & Chen, Q. M. Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol. Rep. 35, 2499–2515 (2016).
Pollard, J. W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 4, 71–78 (2004).
Shimizu, K. et al. Immune suppression and reversal of the suppressive tumor microenvironment. Int. Immunol. 30, 445–454 (2018).
Wu, T. & Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 387, 61–68 (2017).
Diop-Frimpong, B. et al. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl Acad. Sci. USA 108, 2909–2914 (2011).
Niu, Y. M. et al. Size shrinkable drug delivery nanosystems and priming the tumor microenvironment for deep intratumoral penetration of nanoparticles. J. Control. Release 277, 35–47 (2018).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Li, W. & Sun, X. Recent advances in developing novel anti-cancer drugs targeting tumor hypoxic and acidic microenvironments. Recent Pat. Anticancer Drug Discov. 13, 455–468 (2018).
Sharma, A. et al. Hypoxia-targeted drug delivery. Chem. Soc. Rev. 48, 771–813 (2019).
Kessenbrock, K., Plaks, V. & Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
Mahzouni, P. & Sarmadi, T. An observational study on the expression of cyclooxygenase-2 in meningioma. Adv. Biomed. Res. 3, 211 (2014).
Lu, Y., Aimetti, A. A., Langer, R. & Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2, 16075 (2017).
Pelaz, B. et al. Diverse applications of nanomedicine. ACS Nano 11, 2313–2381 (2017).
Golombek, S. K. et al. Tumor targeting via EPR: strategies to enhance patient responses. Adv. Drug Deliv. Rev. 130, 17–38 (2018).
Liu, J. J., Chen, Q., Feng, L. Z. & Liu, Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today 21, 55–73 (2018).
Li, S. et al. Nanoparticle-mediated local depletion of tumour-associated platelets disrupts vascular barriers and augments drug accumulation in tumours. Nat. Biomed. Eng. 1, 667–679 (2017).
Han, X. et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun. 9, 3390 (2018).
Ovais, M., Guo, M. & Chen, C. Tailoring nanomaterials for targeting tumor-associated macrophages. Adv. Mater. 31, e1808303 (2019).
Miao, L. et al. Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Cancer Res. 77, 719–731 (2017).
Ho-Tin-Noe, B., Demers, M. & Wagner, D. D. How platelets safeguard vascular integrity. J. Thromb. Haemost. 9, 56–65 (2011).
Neesse, A., Algul, H., Tuveson, D. A. & Gress, T. M. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64, 1476–1484 (2015).
Demers, M. et al. Increased efficacy of breast cancer chemotherapy in thrombocytopenic mice. Cancer Res. 71, 1540–1549 (2011).
Zhang, Y. L. et al. Inhibition of platelet function using liposomal nanoparticles blocks tumor metastasis. Theranostics 7, 1062–1071 (2017).
Ozdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Ho-Tin-Noe, B. et al. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 68, 6851–6858 (2008).
Volz, J. et al. Inhibition of platelet GPVI induces intratumor hemorrhage and increases efficacy of chemotherapy in mice. Blood 133, 2696–2706 (2019).
Demers, M. & Wagner, D. D. Targeting platelet function to improve drug delivery. Oncoimmunology 1, 100–102 (2012).
Bergmeier, W. et al. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood 95, 886–893 (2000).
Lee, Y. & Thompson, D. H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 9, 1450 (2017).
Meng, H. et al. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 9, 3540–3557 (2015).
Dangi-Garimella, S. et al. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 71, 1019–1028 (2011).
Fan, Y., Li, C., Li, F. & Chen, D. pH-activated size reduction of large compound nanoparticles for in vivo nucleus-targeted drug delivery. Biomaterials 85, 30–39 (2016).
Wang, H. et al. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials 32, 8281–8290 (2011).
Elbakry, A. et al. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 9, 2059–2064 (2009).
Ding, C., Gu, J., Qu, X. & Yang, Z. Preparation of multifunctional drug carrier for tumor-specific uptake and enhanced intracellular delivery through the conjugation of weak acid labile linker. Bioconjug. Chem. 20, 1163–1170 (2009).
Long, A. H. et al. Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol. Res. 4, 869–880 (2016).
Mirza, N. et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 66, 9299–9307 (2006).
Song, W. J. et al. Gold nanoparticles capped with polyethyleneimine for enhanced siRNA delivery. Small 6, 239–246 (2010).
Park, K. M. et al. All-trans-retinoic acid (ATRA)-grafted polymeric gene carriers for nuclear translocation and cell growth control. Biomaterials 30, 2642–2652 (2009).
Swartz, M. A. et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 72, 2473–2480 (2012).
Li, H., Qiu, Z. W., Li, F. & Wang, C. L. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 14, 5865–5870 (2017).
Salem, N. et al. High expression of matrix metalloproteinases: MMP-2 and MMP-9 predicts poor survival outcome in colorectal carcinoma. Future Oncol. 12, 323–331 (2016).
Zheng, H. C. et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 26, 3579–3583 (2006).
Trudel, D. et al. Significance of MMP-2 expression in prostate cancer: an immunohistochemical study. Cancer Res. 63, 8511–8515 (2003).
Han, X. et al. An extendable star-like nanoplatform for functional and anatomical imaging-guided photothermal oncotherapy. ACS Nano 13, 4379–4391 (2019).
Kanamala, M. et al. Dual pH-sensitive liposomes with low pH-triggered sheddable PEG for enhanced tumor-targeted drug delivery. Nanomedicine (Lond). 14, 1972–1990 (2019).
Li, H. J. et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc. Natl Acad. Sci. USA 113, 4164–4169 (2016).
Bachem, M. G. et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115, 421–432 (1998).
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB36000000), the National Key R&D Program of China (2018YFA0208900 and 2018YFE0205300), the K. C. Wong Education Foundation (GJTD-2018-03), the Key Research Program of Frontier Sciences CAS (ZDBS-LY-SLH039), the National Postdoctoral Program for Innovative Talents (BX20180083), and the National Natural Science Foundation of China (31671023).
Author information
Authors and Affiliations
Contributions
Y.Z. and G.N. originated the method of preparing PLP-D-R; X.H. and G.N. originated the method of preparing Au@PP/RA/siHSP47. All authors wrote the manuscript and approved the contents of the protocol.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Giuseppe Curigliano, Zhuang Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Li, S. et al. Nat. Biomed. Eng. 1, 667–679 (2017): https://doi.org/10.1038/s41551-017-0115-8
Han, X. et al. Nat. Commun. 9, 3390 (2018): https://doi.org/10.1038/s41467-018-05906-x
Rights and permissions
About this article
Cite this article
Zhang, Y., Han, X. & Nie, G. Responsive and activable nanomedicines for remodeling the tumor microenvironment. Nat Protoc 16, 405–430 (2021). https://doi.org/10.1038/s41596-020-00421-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00421-0
This article is cited by
-
Pathogenesis-adaptive polydopamine nanosystem for sequential therapy of ischemic stroke
Nature Communications (2023)
-
Identification of potential tumor antigens and immune subtypes for lung adenocarcinoma
Medical Oncology (2023)
-
Nano Phytoceuticals: A Step Forward in Tracking Down Paths for Therapy Against Pancreatic Ductal Adenocarcinoma
Journal of Cluster Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.