Abstract
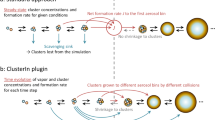
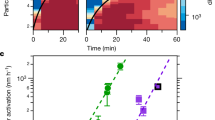
Atmospheric new particle formation (NPF), which is observed in many environments globally, is an important source of boundary-layer aerosol particles and cloud condensation nuclei, which affect both the climate and human health. To better understand the mechanisms behind NPF, chamber experiments can be used to simulate this phenomenon under well-controlled conditions. Recent advancements in instrumentation have made it possible to directly detect the first steps of NPF of molecular clusters (~1–2 nm in diameter) and to calculate quantities such as the formation and growth rates of these clusters. Whereas previous studies reported particle formation rates as the flux of particles across a specified particle diameter or calculated them from measurements of larger particle sizes, this protocol outlines methods to directly quantify particle dynamics for cluster sizes. Here, we describe the instrumentation and analysis methods needed to quantify particle dynamics during NPF of sub-3-nm aerosol particles in chamber experiments. The methods described in this protocol can be used to make results from different chamber experiments comparable. The experimental setup, collection and post-processing of the data, and thus completion of this protocol, take from months up to years, depending on the chamber facility, experimental plan and level of expertise. Use of this protocol requires engineering capabilities and expertise in data analysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Spracklen, D. V. et al. The contribution of boundary layer nucleation events to total particle concentrations on regional and global scales. Atmos. Chem. Phys. 6, 5631–5648 (2006).
Yu, F. et al. Spatial distributions of particle number concentrations in the global troposphere: Simulations, observations, and implications for nucleation mechanisms. J. Geophy. Res. 115, D17205 (2010).
Spracklen, D. V. et al. Contribution of particle formation to global cloud condensation nuclei concentrations. Geophy. Res. Lett. 35, L06808 (2008).
Kerminen, V. M. et al. Cloud condensation nuclei production associated with atmospheric nucleation: a synthesis based on existing literature and new results. Atmos. Chem. Phys. 12, 12037–12059 (2012).
Guo, S. et al. Elucidating severe urban haze formation in China. Proc. Natl Acad. Sci. USA 111, 17373–17378 (2014).
Jiang, J. K. et al. First measurements of neutral atmospheric cluster and 1-2 nm particle number size distributions during nucleation events. Aerosol Sci. Technol. 45, ii–v (2011).
Kulmala, M. et al. Direct observations of atmospheric aerosol nucleation. Science 339, 943–946 (2013).
Zhang, R., Khalizov, A., Wang, L., Hu, M. & Xu, W. Nucleation and growth of nanoparticles in the atmosphere. Chem. Rev. 112, 1957–2011 (2011).
Kerminen, V.-M. et al. Atmospheric new particle formation and growth: review of field observations. Environ. Res. Lett. 13, 103003 (2018).
Chu, B. et al. Atmospheric new particle formation in China. Atmos. Chem. Phys. 19, 115–138 (2019).
Akimoto, H., Sakamaki, F., Hoshino, M., Inoue, G. & Okuda, M. Photochemical ozone formation in propylene-nitrogen oxide-dry air system. Environ. Sci. 13, 53–58 (1979).
Alfarra, M. R. et al. A mass spectrometric study of secondary organic aerosols formed from the photooxidation of anthropogenic and biogenic precursors in a reaction chamber. Atmos. Chem. Phys. 6, 5279–5293 (2006).
Atkinson, R., Carter, W. P. L., Darnall, K. R., Winer, A. M. & Pitts Jr., J. N. A smog chamber and modeling study of the gas phase NOx–air photooxidation of toluene and the cresols. 12, 779-836 (1980).
Barsanti, K. C., McMurry, P. H. & Smith, J. N. The potential contribution of organic salts to new particle growth. Atmos. Chem. Phys. 9, 2949–2957 (2009).
Becker, K. H. Overview on the Development of Chambers for the Study of Atmospheric Chemical Processes (Springer Netherlands, 2006).
Behnke, W., Holländer, W., Koch, W., Nolting, F. & Zetzsch, C. A smog chamber for studies of the photochemical degradation of chemicals in the presence of aerosols. Atmos. Environ. 22, 1113–1120 (1988).
Bruns, E. A. et al. Inter-comparison of laboratory smog chamber and flow reactor systems on organic aerosol yield and composition. Atmos. Meas. Technol. 8, 2315–2332 (2015).
Böge, O., Miao, Y., Plewka, A. & Herrmann, H. Formation of secondary organic particle phase compounds from isoprene gas-phase oxidation products: an aerosol chamber and field study. Atmos. Environ. 40, 2501–2509 (2006).
Carter, W. P. L., Atkinson, R., Winer, A. M. & Pitts, J. N. Jr. Experimental investigation of chamber-dependent radical sources. Int. J. Chem. Kinet. 14, 1071–1103 (1982).
Carter, W. P. L. et al. A new environmental chamber for evaluation of gas-phase chemical mechanisms and secondary aerosol formation. Atmos. Environ. 39, 7768–7788 (2005).
Dodge, M. C. Chemical oxidant mechanisms for air quality modeling: critical review. Atmos. Environ. 34, 2103–2130 (2000).
Donahue, N. M. et al. Aging of biogenic secondary organic aerosol via gas-phase OH radical reactions. Proc. Natl Acad. Sci. USA 109, 13503–13508 (2012).
Ehn, M. et al. A large source of low-volatility secondary organic aerosol. Nature 506, 476–479 (2014).
Hallquist, M. et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 9, 5155–5236 (2009).
Hess, G. D., Carnovale, F., Cope, M. E. & Johnson, G. M. The evaluation of some photochemical smog reaction mechanisms—I. Temperature and initial composition effects. Atmos. Environ. A Gen. Top. 26, 625–641 (1992).
Hoffmann, T. et al. Formation of organic aerosols from the oxidation of biogenic hydrocarbons. J. Atmos. Chem. 26, 189–222 (1997).
Jeffries, H., Kamens, R., Sexron, K. & Gerhardt, A. Outdoor smog chamber experiments to test photochemical models. Final report May 78–May 81 (North Carolina University at Chapel Hill School of Public Health, 1982).
Jimenez, J. L. et al. Evolution of organic aerosols in the atmosphere. Science 326, 1525–1529 (2009).
Kalberer, M., Sax, M. & Samburova, V. Molecular size evolution of oligomers in organic aerosols collected in urban atmospheres and generated in a smog chamber. Environ. Sci. Technol. 40, 5917–5922 (2006).
Kroll, J. H., Ng, N. L., Murphy, S. M., Flagan, R. C. & Seinfeld, J. H. Secondary organic aerosol formation from isoprene photooxidation under high-NOx conditions. Geophys. Res. Lett. 32, L18808 (2005).
Kroll, J. H. et al. Chamber studies of secondary organic aerosol growth by reactive uptake of simple carbonyl compounds. J. Geophys. Res. 110, D23207 (2005).
Leskinen, A. P., Kulmala, M. & Lehtinen, K. E. J. Growth of nucleation mode particles: source rates of condensable vapour in a smog chamber. Atmos. Environ. 42, 7405–7411 (2008).
Martín-Reviejo, M. & Wirtz, K. Is benzene a precursor for secondary organic aerosol? Environ. Sci. Technol. 39, 1045–1054 (2005).
McFiggans, G. et al. Secondary organic aerosol reduced by mixture of atmospheric vapours. Nature 565, 587–593 (2019).
McMurry, P. H. Photochemical aerosol formation from SO2: a theoretical analysis of smog chamber data. J. Colloid Interf. Sci. 78, 513–527 (1980).
Ng, N. L. et al. Secondary organic aerosol formation from m-xylene, toluene, and benzene. Atmos. Chem. Phys. 7, 3909–3922 (2007).
Nordin, E. Z. et al. Secondary organic aerosol formation from idling gasoline passenger vehicle emissions investigated in a smog chamber. Atmos. Chem. Phys. 13, 6101–6116 (2013).
O’Dowd, C. D. et al. Marine aerosol formation from biogenic iodine emissions. Nature 417, 632 (2002).
Odum, J. R. et al. Gas/particle partitioning and secondary organic aerosol yields. Environ. Sci. Technol. 30, 2580–2585 (1996).
Pandis, S. N., Paulson, S. E., Seinfeld, J. H. & Flagan, R. C. Aerosol formation in the photooxidation of isoprene and β-pinene. Atmos. Environ. A Gen. Top. 25, 997–1008 (1991).
Platt, S. M. et al. Secondary organic aerosol formation from gasoline vehicle emissions in a new mobile environmental reaction chamber. Atmos. Chem. Phys. 13, 9141–9158 (2013).
Riva, M. et al. Chemical transformations in monoterpene-derived organic aerosol enhanced by inorganic composition. npj Clim. Atmos. Sci. 2, 2 (2019).
Simonaitis, R., Meagher, J. F. & Bailey, E. M. Evaluation of the condensed carbon bond (CB-IV) mechanism against smog chamber data at low VOC and NOx concentrations. Atmos. Environ. 31, 27–43 (1997).
Smith, J. N. et al. Observations of aminium salts in atmospheric nanoparticles and possible climatic implications. Proc. Natl Acad. Sci. USA 107, 6634–6639 (2010).
Tritscher, T. et al. Volatility and hygroscopicity of aging secondary organic aerosol in a smog chamber. Atmos. Chem. Phys. 11, 11477–11496 (2011).
Wang, X. et al. Design and characterization of a smog chamber for studying gas-phase chemical mechanisms and aerosol formation. Atmos. Meas. Technol. 7, 301–313 (2014).
Weitkamp, E. A., Sage, A. M., Pierce, J. R., Donahue, N. M. & Robinson, A. L. Organic aerosol formation from photochemical oxidation of diesel exhaust in a smog chamber. Environ. Sci. Technol. 41, 6969–6975 (2007).
Zhang, X. et al. Influence of vapor wall loss in laboratory chambers on yields of secondary organic aerosol. Proc. Natl Acad. Sci. USA 111, 5802–7 (2014).
Zhao, J. et al. Observation of neutral sulfuric acid-amine containing clusters in laboratory and ambient measurements. Atmos. Chem. Phys. 11, 10823–10836 (2011).
Garnier, J. P. & Mirabel, P. Experimental study of nucleation in binary mixtures: the methanol–ethanol, methanol‐n‐propanol, and ethanol‐n‐propanol systems. J. Chem. Phys. 77, 2035–2037 (1982).
Viisanen, Y., Kulmala, M. & Laaksonen, A. Experiments on gas–liquid nucleation of sulfuric acid and water. J. Chem. Phys. 107, 920–926 (1997).
Ball, S., Hanson, D., Eisele, F. & McMurry, P. Laboratory studies of particle nucleation: initial results for H2SO4, H2O, and NH3 vapors. J. Geophys. Res-Atmos. 104, 23709–23718 (1999).
Mirabel, P. & Clavelin, J. L. Experimental study of nucleation in binary mixtures: the nitric acid–water and sulfuric acid–water systems. J. Chem. Phys. 68, 5020–5027 (1978).
Kirkby, J. et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature 476, 429–433 (2011).
Dawson, M. L. et al. Simplified mechanism for new particle formation from methanesulfonic acid, amines, and water via experiments and ab initio calculations. Proc. Natl Acad. Sci. USA 109, 18719–18724 (2012).
Ezell, M. et al. A new aerosol flow system for photochemical and thermal studies of tropospheric aerosols. Aerosol Sci. Technol. 44, 329–338 (2010).
Jimenez, J. L. et al. New particle formation from photooxidation of diiodomethane (CH2I2). J. Geophys. Res. 108, 4318 (2003).
Zhang, R. et al. Atmospheric new particle formation enhanced by organic acids. Science 304, 1487–1490 (2004).
Berndt, T., Böge, O., Stratmann, F., Heintzenberg, J. & Kulmala, M. Rapid formation of sulfuric acid particles at near-atmospheric conditions. Science 307, 698–700 (2005).
Berndt, T., Böge, O. & Stratmann, F. Formation of atmospheric H2SO4/H2O particles in the absence of organics: a laboratory study. Geophys. Res. Lett. 33, L15817 (2006).
Benson, D. R., Young, L.-H., Kameel, F. R. & Lee, S.-H. Laboratory-measured nucleation rates of sulfuric acid and water binary homogeneous nucleation from the SO2 + OH reaction. Geophys. Res. Lett. 35, L11801 (2008).
Berndt, T. et al. SO2 oxidation products other than H2SO4 as a trigger of new particle formation. Part 1: laboratory investigations. Atmos. Chem. Phys. 8, 6365–6374 (2008).
Young, L. H. et al. Laboratory studies of H2SO4/H2O binary homogeneous nucleation from the SO2+OH reaction: evaluation of the experimental setup and preliminary results. Atmos. Chem. Phys. 8, 4997–5016 (2008).
Berndt, T. et al. Laboratory study on new particle formation from the reaction OH + SO2: influence of experimental conditions, H2O vapour, NH3 and the amine tert-butylamine on the overall process. Atmos. Chem. Phys. 10, 7101–7116 (2010).
Brus, D., Hyvärinen, A. P., Viisanen, Y., Kulmala, M. & Lihavainen, H. Homogeneous nucleation of sulfuric acid and water mixture: experimental setup and first results. Atmos. Chem. Phys. 10, 2631–2641 (2010).
Duplissy, J. et al. Results from the CERN pilot CLOUD experiment. Atmos. Chem. Phys. 10, 1635–1647 (2010).
Sipilä, M. et al. The role of sulfuric acid in atmospheric nucleation. Science 327, 1243–1246 (2010).
Wang, L. et al. Atmospheric nanoparticles formed from heterogeneous reactions of organics. Nat. Geosci. 3, 238 (2010).
Brus, D. et al. Homogenous nucleation of sulfuric acid and water at close to atmospherically relevant conditions. Atmos. Chem. Phys. 11, 5277–5287 (2011).
Kiendler-Scharr, A. et al. New particle formation in forests inhibited by isoprene emissions. Nature 461, 381–384 (2009).
Benson, D. R., Yu, J. H., Markovich, A. & Lee, S. H. Ternary homogeneous nucleation of H2SO4, NH3, and H2O under conditions relevant to the lower troposphere. Atmos. Chem. Phys. 11, 4755–4766 (2011).
Yu, H., McGraw, R. & Lee, S.-H. Effects of amines on formation of sub-3 nm particles and their subsequent growth. Geophys. Res. Lett. 39, L02807 (2012).
Zollner, J. H. et al. Sulfuric acid nucleation: power dependencies, variation with relative humidity, and effect of bases. Atmos. Chem. Phys. 12, 4399–4411 (2012).
Almeida, J. et al. Molecular understanding of sulphuric acid-amine particle nucleation in the atmosphere. Nature 502, 359–363 (2013).
Schobesberger, S. et al. Molecular understanding of atmospheric particle formation from sulfuric acid and large oxidized organic molecules. Proc. Natl Acad. Sci. USA 110, 17223–17228 (2013).
Jen, C. N., McMurry, P. H. & Hanson, D. R. Stabilization of sulfuric acid dimers by ammonia, methylamine, dimethylamine, and trimethylamine. J. Geophys. Res. Atmos. 119, 7502–7514 (2014).
Riccobono, F. et al. Oxidation products of biogenic emissions contribute to nucleation of atmospheric particles. Science 344, 717–721 (2014).
Glasoe, W. A. et al. Sulfuric acid nucleation: an experimental study of the effect of seven bases. J. Geophys. Res. Atmos. 120, 1933–1950 (2015).
Chen, H. et al. New particle formation and growth from methanesulfonic acid, trimethylamine and water. Phys. Chem. Chem. Phys. 17, 13699–13709 (2015).
Chen, H., Varner, M. E., Gerber, R. B. & Finlayson-Pitts, B. J. Reactions of methanesulfonic acid with amines and ammonia as a source of new particles in air. J. Phys. Chem. B 120, 1526–1536 (2016).
Jen, C. N., Bachman, R., Zhao, J., McMurry, P. H. & Hanson, D. R. Diamine-sulfuric acid reactions are a potent source of new particle formation. Geophys. Res. Lett. 43, 867–873 (2016).
Lehtipalo, K. et al. The effect of acid-base clustering and ions on the growth of atmospheric nano-particles. Nat. Commun. 7, 11594 (2016).
Yu, H. et al. Laboratory observations of temperature and humidity dependencies of nucleation and growth rates of sub-3 nm particles. J. Geophys. Res. Atmos. 122, 1919–1929 (2017).
Chen, H. & Finlayson-Pitts, B. J. New particle formation from methanesulfonic acid and amines/ammonia as a function of temperature. Environ. Sci. Technol. 51, 243–252 (2017).
Tröstl, J. et al. The role of low-volatility organic compounds in initial particle growth in the atmosphere. Nature 533, 527–531 (2016).
Dal Maso, M. et al. A chamber study of the influence of boreal BVOC emissions and sulfuric acid on nanoparticle formation rates at ambient concentrations. Atmos. Chem. Phys. 16, 1955–1970 (2016).
Boulon, J. et al. Sub-3 nm particles detection in a large photoreactor background: possible implications for new particles formation studies in a smog chamber. Aerosol Sci. Technol. 47, 153–157 (2013).
Wang, J. et al. Design of a new multi-phase experimental simulation chamber for atmospheric photosmog, aerosol and cloud chemistry research. Atmos. Meas. Technol. 4, 2465 (2011).
Pichelstorfer, L. et al. Resolving nanoparticle growth mechanisms from size- and time-dependent growth rate analysis. Atmos. Chem. Phys. 18, 1307–1323 (2018).
Kürten, A. et al. New particle formation in the sulfuric acid-dimethylamine-water system: reevaluation of CLOUD chamber measurements and comparison to an aerosol nucleation and growth model. Atmos. Chem. Phys. 18, 845–863 (2018).
Hao, L. Q. et al. New particle formation from the oxidation of direct emissions of pine seedlings. Atmos. Chem. Phys. 9, 8121–8137 (2009).
Joutsensaari, J. et al. Nanoparticle formation by ozonolysis of inducible plant volatiles. Atmos. Chem. Phys. 5, 1489–1495 (2005).
Paulsen, D. et al. Secondary organic aerosol formation by irradiation of 1, 3, 5-trimethylbenzene−NOx−H2O in a new reaction chamber for atmospheric chemistry and physics. Environ. Sci. Technol. 39, 2668–2678 (2005).
Metzger, A. et al. Evidence for the role of organics in aerosol particle formation under atmospheric conditions. Proc. Natl Acad. Sci. USA 107, 6646–6651 (2010).
Riccobono, F. et al. Contribution of sulfuric acid and oxidized organic compounds to particle formation and growth. Atmos. Chem. Phys. 12, 9427–9439 (2012).
Duplissy, J. et al. Effect of ions on sulfuric acid-water binary particle formation: 2. Experimental data and comparison with QC-normalized classical nucleation theory. J. Geophys. Res. Atmos. 121, 1752–1775 (2016).
Wagner, R. et al. The role of ions in new particle formation in the CLOUD chamber. Atmos. Chem. Phys. 17, 15181–15197 (2017).
Kirkby, J. et al. Ion-induced nucleation of pure biogenic particles. Nature 533, 521–526 (2016).
Smith, J. N., Moore, K. F., McMurry, P. H. & Eisele, F. L. Atmospheric measurements of sub-20 nm diameter particle chemical composition by thermal desorption chemical ionization mass spectrometry. Aerosol Sci. Technol. 38, 100–110 (2004).
Smith, J. N., Winkler, P. M., Zhao, J. & McMurry, P. H. Exploring the role of organics in atmospheric new particle formation with chemical ionization mass spectrometry. Abstr. Pap. Am. Chem. Soc. 242, ENVR 428 (2011).
Smith, J. N. & Rathbone, G. J. Carboxylic acid characterization in nanoparticles by thermal desorption chemical ionization mass spectrometry. Int. J. Mass. Spectrom. 274, 8–13 (2008).
Lehtipalo, K. et al. Multicomponent new particle formation from sulfuric acid, ammonia, and biogenic vapors. Sci. Adv. 4, eaau5363 (2018).
Dunne, E. M. et al. Global atmospheric particle formation from CERN CLOUD measurements. Science 354, 1119–1124 (2016).
Gordon, H. et al. Causes and importance of new particle formation in the present-day and preindustrial atmospheres. J. Geophys. Res. Atmos. 122, 8739–8760 (2017).
Gordon, H. et al. Reduced anthropogenic aerosol radiative forcing caused by biogenic new particle formation. Proc. Natl Acad. Sci. USA 113, 12053–12058 (2016).
Cziczo, D. J. et al. Ice nucleation by surrogates of Martian mineral dust: what can we learn about Mars without leaving Earth? 118, 1945-1954 (2013).
Berndt, T. et al. Enhancement of atmospheric H2SO4/H2O nucleation: organic oxidation products versus amines. Atmos. Chem. Phys. 14, 751–764 (2014).
McMurry, P. H. & Grosjean, D. Gas and aerosol wall losses in Teflon film smog chambers. Environ. Sci. Technol. 19, 1176–1182 (1985).
Liu, D.-L. in Developments in Surface Contamination and Cleaning (eds Kohli, R. & Mittal, K. L.) 1–56 (William Andrew Publishing, 2010).
Schwantes, R. H. et al. in Advances in Atmospheric Chemistry 1–93 (World Scientific, 2017).
Neitola, K. et al. Total sulfate vs. sulfuric acid monomer concenterations in nucleation studies. Atmos. Chem. Phys. 15, 3429–3443 (2015).
Stolzenburg, D. et al. Rapid growth of organic aerosol nanoparticles over a wide tropospheric temperature range. Proc. Natl Acad. Sci. USA 115, 9122–9127 (2018).
Wildt, J. et al. Suppression of new particle formation from monoterpene oxidation by NOx. Atmos. Chem. Phys. 14, 2789–2804 (2014).
Kulmala, M. et al. Measurement of the nucleation of atmospheric aerosol particles. Nat. Protoc. 7, 1651–1667 (2012).
Kerminen, V. M. & Kulmala, M. Analytical formulae connecting the “real” and the “apparent” nucleation rate and the nuclei number concentration for atmospheric nucleation events. J. Aerosol Sci. 33, 609–622 (2002).
Lehtinen, K. E. J., Dal Maso, M., Kulmala, M. & Kerminen, V. M. Estimating nucleation rates from apparent particle formation rates and vice versa: revised formulation of the Kerminen-Kulmala equation. J. Aerosol Sci. 38, 988–994 (2007).
Korhonen, H., Kerminen, V.-M., Kokkola, H. & Lehtinen, K. E. J. Estimating atmospheric nucleation rates from size distribution measurements: analytical equations for the case of size dependent growth rates. J. Aerosol Sci. 69, 13–20 (2014).
Kürten, A., Williamson, C., Almeida, J., Kirkby, J. & Curtius, J. On the derivation of particle nucleation rates from experimental formation rates. Atmos. Chem. Phys. 15, 4063–4075 (2015).
Brines, M. et al. Traffic and nucleation events as main sources of ultrafine particles in high-insolation developed world cities. Atmos. Chem. Phys. 15, 5929–5945 (2015).
Cai, R. et al. Estimating the influence of transport on aerosol size distributions during new particle formation events. Atmos. Chem. Phys. 18, 16587–16599 (2018).
Dada, L. et al. Refined classification and characterization of atmospheric new-particle formation events using air ions. Atmos. Chem. Phys. 18, 17883–17893 (2018).
Leino, K. et al. Vertical profiles of sub-3 nm particles over the boreal forest. Atmos. Chem. Phys. 19, 4127–4138 (2019).
Kerminen, V. M., Lehtinen, K. E. J., Anttila, T. & Kulmala, M. Dynamics of atmospheric nucleation mode particles: a timescale analysis. Tellus B 56, 135–146 (2004).
Cai, R., Mirme, S., Jiang, J. & Kangasluoma, J. Parameters to determine the optimum performance of electrical mobility spectrometers for measurement of particle size distributions down to the cluster size. J. Aerosol Sci. 127, 102–115 (2018).
Mordas, G. et al. Design and performance characteristics of a condensation particle counter UF-02proto. Boreal Environ. Res. 10, 543–552 (2005).
Collins, A. M., Dick, W. D. & Romay, F. J. A new coincidence correction method for condensation particle counters. Aerosol Sci. Technol. 47, 177–182 (2013).
Kebabian, P. L., Herndon, S. C. & Freedman, A. Detection of nitrogen dioxide by cavity attenuated phase shift spectroscopy. Anal. Chem. 77, 724–728 (2005).
Rohrer, F. & Brüning, D. Surface NO and NO2 mixing ratios measured between 30 N and 30 S in the Atlantic region. J. Atmos. Chem. 15, 253–267 (1992).
Hansel, A. et al. Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. Int. J. Mass. Spectrom. 149, 609–619 (1995).
Blake, R. S., Monks, P. S. & Ellis, A. M. Proton-transfer reaction mass spectrometry. Chem. Rev. 109, 861–896 (2009).
Yuan, B. et al. Proton-transfer-reaction mass spectrometry: applications in atmospheric sciences. Chem. Rev. 117, 13187–13229 (2017).
Jokinen, T. et al. Atmospheric sulphuric acid and neutral cluster measurements using CI-APi-TOF. Atmos. Chem. Phys. 12, 4117–4125 (2012).
Kürten, A., Rondo, L., Ehrhart, S. & Curtius, J. Calibration of a chemical ionization mass spectrometer for the measurement of gaseous sulfuric acid. Phys. Chem. A 116, 6375–6386 (2012).
Heinritzi, M. et al. Characterization of the mass-dependent transmission efficiency of a CIMS. Atmos. Meas. Technol. 9, 1449–1460 (2016).
Lee, B. H. et al. An iodide-adduct high-resolution time-of-flight chemical-ionization mass spectrometer: application to atmospheric inorganic and organic compounds. Environ. Sci. Technol. 48, 6309–6317 (2014).
Brophy, P. & Farmer, D. K. Clustering, methodology, and mechanistic insights into acetate chemical ionization using high-resolution time-of-flight mass spectrometry. Atmos. Meas. Technol. 9, 3969–3986 (2016).
Breitenlechner, M. et al. PTR3: an instrument for studying the lifecycle of reactive organic carbon in the atmosphere. Anal. Chem. 89, 5824–5831 (2017).
Krechmer, J. et al. Evaluation of a new reagent-ion source and focusing ion–molecule reactor for use in proton-transfer-reaction mass spectrometry. Anal. Chem. 90, 12011–12018 (2018).
Yao, L. et al. Detection of atmospheric gaseous amines and amides by a high-resolution time-of-flight chemical ionization mass spectrometer with protonated ethanol reagent ions. Atmos. Chem. Phys. 16, 14527–14543 (2016).
Zheng, J. et al. Measurement of atmospheric amines and ammonia using the high resolution time-of-flight chemical ionization mass spectrometry. Atmos. Environ. 102, 249–259 (2015).
Simon, M. et al. Detection of dimethylamine in the low pptv range using nitrate chemical ionization atmospheric pressure interface time-of-flight (CI-APi-TOF) mass spectrometry. Atmos. Meas. Technol. 9, 2135–2145 (2016).
Praplan, A. P., Bianchi, F., Dommen, J. & Baltensperger, U. Dimethylamine and ammonia measurements with ion chromatography during the CLOUD4 campaign. Atmos. Meas. Technol. 5, 2161–2167 (2012).
Junninen, H. et al. A high-resolution mass spectrometer to measure atmospheric ion composition. Atmo. s. Meas. Technol. 3, 1039–1053 (2010).
Frege, C. et al. Influence of temperature on the molecular composition of ions and charged clusters during pure biogenic nucleation. Atmos. Chem. Phys. 18, 65–79 (2018).
Bianchi, F. et al. The role of highly oxygenated molecules (HOMs) in determining the composition of ambient ions in the boreal forest. Atmos. Chem. Phys. 17, 13819–13831 (2017).
Ehn, M. et al. Composition and temporal behavior of ambient ions in the boreal forest. Atmos. Chem. Phys. 10, 8513–8530 (2010).
Loza, C. L. et al. Characterization of vapor wall loss in laboratory chambers. Environ. Sci. Technol. 44, 5074–5078 (2010).
Brauers, T. et al. Investigation of the formaldehyde differential absorption cross section at high and low spectral resolution in the simulation chamber SAPHIR. Atmos. Chem. Phys. 7, 3579–3586 (2007).
Sumner, A. L. et al. in Dynamics of Mercury Pollution on Regional and Global Scales: Atmospheric Processes and Human Exposures Around the World (eds Pirrone, N. & Mahaffey, K. R.) 193–212 (Springer, 2005).
Grieshop, A. P., Logue, J. M., Donahue, N. M. & Robinson, A. L. Laboratory investigation of photochemical oxidation of organic aerosol from wood fires 1: measurement and simulation of organic aerosol evolution. Atmos. Chem. Phys. 9, 1263–1277 (2009).
Byrne, M. A., Goddard, A. J. H., Lange, C. & Roed, J. Stable tracer aerosol deposition measurements in a test chamber. J. Aerosol Sci. 26, 645–653 (1995).
Presto, A. A., Gordon, T. D. & Robinson, A. L. Primary to secondary organic aerosol: evolution of organic emissions from mobile combustion sources. Atmos. Chem. Phys. 14, 5015–5036 (2014).
Hunter, J. F., Carrasquillo, A. J., Daumit, K. E. & Kroll, J. H. Secondary organic aerosol formation from acyclic, monocyclic, and polycyclic alkanes. Environ. Sci. Technol. 48, 10227–10234 (2014).
Chhabra, P. S., Flagan, R. C. & Seinfeld, J. H. Elemental analysis of chamber organic aerosol using an aerodyne high-resolution aerosol mass spectrometer. Atmos. Chem. Phys. 10, 4111–4131 (2010).
Saathoff, H. et al. Temperature dependence of yields of secondary organic aerosols from the ozonolysis of α-pinene and limonene. Atmos. Chem. Phys. 9, 1551–1577 (2009).
Ye, P. et al. Vapor wall loss of semi-volatile organic compounds in a Teflon chamber. Aerosol Sci. Technol. 50, 822–834 (2016).
Carter, W. P. L., Heo, G., Cocker III, D. R. & Nakao, S. SOA formation: chamber study and model development. Final report to the California Air Resources Board, contract no. 08-326 https://intra.engr.ucr.edu/~carter/SAPRC/pmchrpt.pdf (2012).
Cocker, D. R., Flagan, R. C. & Seinfeld, J. H. State-of-the-art chamber facility for studying atmospheric aerosol chemistry. Environ. Sci. Technol. 35, 2594–2601 (2001).
McMurry, P. H. & Rader, D. J. Aerosol wall losses in electrically charged chambers. Aerosol Sci. Technol. 4, 249–268 (1985).
Bloss, C. et al. Development of a detailed chemical mechanism (MCMv3.1) for the atmospheric oxidation of aromatic hydrocarbons. Atmos. Chem. Phys. 5, 641–664 (2005).
Saathoff, H. et al. The AIDA soot aerosol characterisation campaign 1999. J. Aerosol Sci. 34, 1277–1296 (2003).
Kulkarni, P., Baron, P. A. & Willeke, K. Aerosol Measurement: Principles, Techniques, and Applications (Wiley, 2011).
Ezell, M. J. et al. A new aerosol flow system for photochemical and thermal studies of tropospheric aerosols. Aerosol Sci. Technol. 44, 329–338 (2010).
Stratmann, F. et al. Laboratory studies and numerical simulations of cloud droplet formation under realistic supersaturation conditions. J. Atmos. Ocean Technol. 21, 876–887 (2004).
Lehtinen, K. E. J. & Kulmala, M. A model for particle formation and growth in the atmosphere with molecular resolution in size. Atmos. Chem. Phys. 3, 251–257 (2003).
Lehtipalo, K. et al. Methods for determining particle size distribution and growth rates between 1 and 3 nm using the Particle Size Magnifier. Boreal Environ. Res. 19, 215–236 (2014).
Kuang, C. et al. Size and time-resolved growth rate measurements of 1 to 5 nm freshly formed atmospheric nuclei. Atmos. Chem. Phys. 12, 3573–3589 (2012).
Lehtinen, K. E. J., Rannik, Ü., Petäjä, T., Kulmala, M. & Hari, P. Nucleation rate and vapor concentration estimations using a least squares aerosol dynamics method. J. Geophys. Res. Atmos. 109, D21209 (2004).
Verheggen, B. & Mozurkewich, M. An inverse modeling procedure to determine particle growth and nucleation rates from measured aerosol size distributions. Atmos. Chem. Phys. 6, 2927–2942 (2006).
Yli-Juuti, T. et al. Growth rates of nucleation mode particles in Hyytiälä during 2003–2009: variation with particle size, season, data analysis method and ambient conditions. Atmos. Chem. Phys. 11, 12865–12886 (2011).
Leppä, J., Anttila, T., Kerminen, V. M., Kulmala, M. & Lehtinen, K. E. J. Atmospheric new particle formation: real and apparent growth of neutral and charged particles. Atmos. Chem. Phys. 11, 4939–4955 (2011).
Li, C. & McMurry, P. H. Errors in nanoparticle growth rates inferred from measurements in chemically reacting aerosol systems. Atmos. Chem. Phys. 18, 8979–8993 (2018).
Vanhanen, J. et al. Particle size magnifier for nano-CN detection. Aerosol Sci. Technol. 45, 533–542 (2011).
Stolzenburg, M. R. & McMurry, P. H. An ultrafine aerosol condensation nucleus counter. Aerosol Sci. Technol. 14, 48–65 (1991).
Hering, S. V. et al. Detection near 1-nm with a laminar-flow, water-based condensation particle counter. Aerosol Sci. Technol. 51, 354-362 (2017).
Wimmer, D. et al. Performance of diethylene glycol-based particle counters in the sub-3 nm size range. Atmos. Meas. Technol. 6, 1793–1804 (2013).
Wang, S. C. & Flagan, R. C. Scanning electrical mobility spectrometer. Aerosol Sci. Technol. 13, 230–240 (1990).
Mirme, S. & Mirme, A. The mathematical principles and design of the NAIS—a spectrometer for the measurement of cluster ion and nanometer aerosol size distributions. Atmos. Meas. Technol. 6, 1061–1071 (2013).
Stolzenburg, D., Steiner, G. & Winkler, P. M. A DMA-train for precision measurement of sub-10 nm aerosol dynamics. Atmos. Meas. Technol. 10, 1639–1651 (2017).
Jiang, J. K., Chen, M. D., Kuang, C. A., Attoui, M. & McMurry, P. H. Electrical mobility spectrometer using a diethylene glycol condensation particle counter for measurement of aerosol size distributions down to 1 nm. Aerosol Sci. Technol. 45, 510–521 (2011).
Kangasluoma, J. et al. Heterogeneous nucleation onto ions and neutralized ions: insights into sign-preference. J. Phys. Chem. C. 120, 7444–7450 (2016).
Kangasluoma, J. et al. Sub-3 nm particle size and composition dependent response of a nano-CPC battery. Atmos. Meas. Technol. 7, 689–700 (2014).
Winkler, P. M. et al. Heterogeneous nucleation experiments bridging the scale from molecular ion clusters to nanoparticles. Science 319, 1374–1377 (2008).
Kupc, A. et al. Laboratory characterization of a new nano-water-based CPC 3788 and performance comparison to an ultrafine butanol-based CPC 3776. Aerosol Sci. Technol. 47, 183–191 (2013).
Kulmala, M. et al. The condensation particle counter battery (CPCB): a new tool to investigate the activation properties of nanoparticles. J. Aerosol Sci. 38, 289–304 (2007).
Kangasluoma, J. & Kontkanen, J. On the sources of uncertainty in the sub-3 nm particle concentration measurement. J. Aerosol Sci. 112, 34–51 (2017).
Wimmer, D. et al. Technical note: using DEG-CPCs at upper tropospheric temperatures. Atmos. Chem. Phys. 15, 7547–7555 (2015).
Gormley, P. G. & Kennedy, M. Diffusion from a stream flowing through a cylindrical tube. Proc. R. Ir. Acad. A Math. Phys. Sci. 52, 163–169 (1949).
Kangasluoma, J. et al. Operation of the Airmodus A11 nano Condensation Nucleus Counter at various inlet pressures and various operation temperatures, and design of a new inlet system. Atmos. Meas. Technol. 9, 2977–2988 (2016).
Fu, Y., Xue, M., Cai, R., Kangasluoma, J. & Jiang, J. Theoretical and experimental analysis of the core sampling method: reducing diffusional losses in aerosol sampling line. Aerosol Sci. Technol. 53, 793–801 (2019).
Seinfeld, J. H. & Pandis, S. N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change (Wiley, 2012).
Poling, B. E., Prausnitz, J. M. & O’Connell, J. P. The Properties of Gases and Liquids 5 (McGraw-Hill, 2001).
Seinfeld, J. H. & Pandis, S. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd edn (Wiley, 2016).
Mikkonen, S. et al. Technical note: effects of uncertainties and number of data points on line fitting—a case study on new particle formation. Atmos. Chem. Phys. 19, 12531–12543 (2019).
Press, W. H., Flannery, B. P., Teukolsky, S. & Vettering, W. T. Numerical Recipes—The Art of Scientific Computing (Cambridge University Press, 1986).
Tellinghuisen, A. Monte Carlo study of precision, bias, inconsistency, and non-gaussian distributions in nonlinear least squares. J. Phys. Chem. A 104, 2834–2844 (2000).
Manninen, H. E. et al. Long-term field measurements of charged and neutral clusters using neutral cluster and air ion spectrometer (NAIS). Boreal Environ. Res. 14, 591–605 (2009).
Bates, D. in Advances in Atomic and Molecular Physics Vol. 20 1–40 (Elsevier, 1985).
Franchin, A. et al. Experimental investigation of ion-ion recombination under atmospheric conditions. Atmos. Chem. Phys. 15, 7203–7216 (2015).
Hoppel, W. A. & Frick, G. M. Ion aerosol attachment coefficients and the steady-state charge-distribution on aerosols in a bipolar ion environment. Aerosol Sci. Technol. 5, 1–21 (1986).
Hering, S. V., Stolzenburg, M. R., Quant, F. R., Oberreit, D. R. & Keady, P. B. A laminar-flow, water-based condensation particle counter (WCPC). Aerosol Sci. Technol. 39, 659–672 (2005).
Mirme, A. et al. A wide-range multi-channel air ion spectrometer. Boreal Environ. Res. 12, 247–264 (2007).
Kürten, A. et al. Neutral molecular cluster formation of sulfuric acid-dimethylamine observed in real time under atmospheric conditions. Proc. Natl Acad. Sci. USA 111, 15019–15024 (2014).
Tiszenkel, L. et al. Temperature effects on sulfuric acid aerosol nucleation and growth: initial results from the TANGENT study. Atmos. Chem. Phys. 19, 8915–8929 (2019).
Benson, D. R., Erupe, M. E. & Lee, S.-H. Laboratory-measured H2SO4-H2O-NH3 ternary homogeneous nucleation rates: initial observations. Geophys. Res. Lett. 36, L15818 (2009).
Erupe, M. E., Viggiano, A. A. & Lee, S. H. The effect of trimethylamine on atmospheric nucleation involving H2SO4. Atmos. Chem. Phys. 11, 4767–4775 (2011).
Krasnomowitz, J. M. et al. Growth of Aitken mode ammonium sulfate particles by α-pinene ozonolysis. Aerosol Sci. Technol. 53, 406–418 (2019).
Stangl, C. M. et al. Sulfur dioxide modifies aerosol particle formation and growth by ozonolysis of monoterpenes and isoprene. J. Geophys. Res. Atmos. 124, 4800–4811 (2019).
Acknowledgements
The CLOUD community is gratefully acknowledged for invaluable discussions. Partial funding was provided by the Academy of Finland (project nos. 316114 and 325647). The work was also supported by the Academy of Finland via the BioFuture2025 project ‘Nano BioMass’, an Academy professor project of M.K. and the Center of Excellence in Atmospheric Sciences (project no. 307331), the European Commission via ACTRIS2 (project no. 654109) and the European Research Council via advanced grant ATM-GTP (project no. 742206).
Author information
Authors and Affiliations
Contributions
L.D., K. Lehtipalo, J. Kontkanen, T.N., K. Lehtinen, V.-M.K. and M.K. contributed to the development of the technique for calculating Jdp and GR. R.B., L.A., J.D., T.P., C.Y., B.C. and J. Kangasluoma contributed to development of the technique for calibrating and minimizing losses during particle measurement. All authors contributed to the writing of this protocol and to the scientific discussions related to it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Véronique Riffault and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Lehtipalo, K. et al. Sci. Adv. 4, eaau5363 (2018): https://doi.org/10.1126/sciadv.aau5363
Wagner, R. et al. Atmos. Chem. Phys. 17, 15181–15197 (2017): https://doi.org/10.5194/acp-17-15181-2017
Supplementary information
Rights and permissions
About this article
Cite this article
Dada, L., Lehtipalo, K., Kontkanen, J. et al. Formation and growth of sub-3-nm aerosol particles in experimental chambers. Nat Protoc 15, 1013–1040 (2020). https://doi.org/10.1038/s41596-019-0274-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0274-z
This article is cited by
-
Aerosol Alteration of Behavioral Response to Pheromone in Bombyx mori
Journal of Chemical Ecology (2023)
-
Terpene emissions from boreal wetlands can initiate stronger atmospheric new particle formation than boreal forests
Communications Earth & Environment (2022)
-
Synergistic HNO3–H2SO4–NH3 upper tropospheric particle formation
Nature (2022)
-
Distribution of non-spherical nanoparticles in turbulent flow of ventilation chamber considering fluctuating particle number density
Applied Mathematics and Mechanics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.