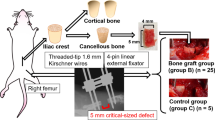
Abstract
Critical-size bone defects, which require large-volume tissue reconstruction, remain a clinical challenge. Bone engineering has the potential to provide new treatment concepts, yet clinical translation requires anatomically and physiologically relevant preclinical models. The ovine critical-size long-bone defect model has been validated in numerous studies as a preclinical tool for evaluating both conventional and novel bone-engineering concepts. With sufficient training and experience in large-animal studies, it is a technically feasible procedure with a high level of reproducibility when appropriate preoperative and postoperative management protocols are followed. The model can be established by following a procedure that includes the following stages: (i) preoperative planning and preparation, (ii) the surgical approach, (iii) postoperative management, and (iv) postmortem analysis. Using this model, full results for peer-reviewed publication can be attained within 2 years. In this protocol, we comprehensively describe how to establish proficiency using the preclinical model for the evaluation of a range of bone defect reconstruction options.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


















Similar content being viewed by others
Data availability
The datasets that support this study are available from the corresponding author upon request.
References
Henkel, J. et al. Bone regeneration based on tissue engineering conceptions – a 21st century perspective. Bone Res. 1, 216–248 (2013).
Weiss, R. J. et al. Decreasing incidence of tibial shaft fractures between 1998 and 2004: information based on 10,627 Swedish inpatients. Acta Orthop. 79, 526–533 (2008).
Wagels, M., Rowe, D., Senewiratne, S., Read, T. & Theile, D. R. Soft tissue reconstruction after compound tibial fracture: 235 cases over 12 years. J. Plast. Reconstr. Aesthet. Surg. 68, 1276–1285 (2015).
Wagels, M., Rowe, D., Senewiratne, S. & Theile, D. R. History of lower limb reconstruction after trauma. ANZ J. Surg. 83, 348–353 (2013).
Sparks, D. S. et al. Vascularised bone transfer: history, blood supply and contemporary problems. J. Plast. Reconstr. Aesthet. Surg. 70, 1–11 (2017).
Reichert, J. C. et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 4, 141ra193 (2012).
Schmitz, J. P. & Hollinger, J. O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 299-308 (1986).
Berner, A. et al. Treatment of long bone defects and non-unions: from research to clinical practice. Cell Tissue Res. 347, 501–519 (2012).
Reichert, J. C. et al. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 30, 2149–2163 (2009).
Balogh, Z. J. et al. Advances and future directions for management of trauma patients with musculoskeletal injuries. Lancet 380, 1109–1119 (2012).
Sparks, D. S., Wagels, M. & Taylor, G. I. Bone reconstruction: a history of vascularized bone transfer. Microsurgery 38, 7–13 (2018).
Cipitria, A. et al. Polycaprolactone scaffold and reduced rhBMP-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 34, 9960–9968 (2013).
Berner, A. et al. Biomimetic tubular nanofiber mesh and platelet rich plasma-mediated delivery of BMP-7 for large bone defect regeneration. Cell Tissue Res. 347, 603–612 (2012).
Reichert, J. C. et al. Custom-made composite scaffolds for segmental defect repair in long bones. Int. Orthop. 35, 1229–1236 (2011).
Berner, A. et al. Effects of scaffold architecture on cranial bone healing. Int. J. Oral. Maxillofac. Surg. 43, 506–513 (2014).
Breschi, A., Gingeras, T. R. & Guigo, R. Comparative transcriptomics in human and mouse. Nat. Rev. Genet. 18, 425–440 (2017).
Wancket, L. M. Animal models for evaluation of bone implants and devices: comparative bone structure and common model uses. Vet. Pathol. 52, 842–850 (2015).
Aerssens, J., Boonen, S., Lowet, G. & Dequeker, J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology 139, 663–670 (1998).
Wang, X., Mabrey, J. D. & Agrawal, C. M. An interspecies comparison of bone fracture properties. Biomed. Mater. Eng. 8, 1–9 (1998).
Reichert, J. C. et al. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng. Part B Rev. 16, 93–104 (2010).
McGovern, J. A., Griffin, M. & Hutmacher, D. W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 11, 33084 (2018).
Pearce, A. I., Richards, R. G., Milz, S., Schneider, E. & Pearce, S. G. Animal models for implant biomaterial research in bone: a review. Eur. Cell. Mater. 13, 1–10 (2007).
Martini, L., Fini, M., Giavaresi, G. & Giardino, R. Sheep model in orthopedic research: a literature review. Comp. Med. 51, 292–299 (2001).
Hu, Y., Zhang, C., Zhang, S., Xiong, Z. & Xu, J. Development of a porous poly(L-lactic acid)/hydroxyapatite/collagen scaffold as a BMP delivery system and its use in healing canine segmental bone defect. J. Biomed. Mater. Res. A 67, 591–598 (2003).
Theyse, L. F., Oosterlaken-Dijksterhuis, M. A., van Doorn, J., Dhert, W. J. & Hazewinkel, H. A. Growth hormone stimulates bone healing in a critical-sized bone defect model. Clin. Orthop. Relat. Res. 446, 259–267 (2006).
Tiedeman, J. J., Lippiello, L., Connolly, J. F. & Strates, B. S. Quantitative roentgenographic densitometry for assessing fracture healing. Clin. Orthop. Relat. Res. 1990, 279-286 (1990).
Takigami, H. et al. Bone formation following OP-1 implantation is improved by addition of autogenous bone marrow cells in a canine femur defect model. J. Orthop. Res. 25, 1333–1342 (2007).
Welter, J. F. et al. Cyclosporin A and tissue antigen matching in bone transplantation. Fibular allografts studied in the dog. Acta Orthop. Scand. 61, 517–527 (1990).
Pek, Y. S., Gao, S., Arshad, M. S., Leck, K. J. & Ying, J. Y. Porous collagen-apatite nanocomposite foams as bone regeneration scaffolds. Biomaterials 29, 4300–4305 (2008).
Meinig, R. P., Buesing, C. M., Helm, J. & Gogolewski, S. Regeneration of diaphyseal bone defects using resorbable poly(L/DL-lactide) and poly(D-lactide) membranes in the Yucatan pig model. J. Orthop. Trauma 11, 551–558 (1997).
Anderson, M. L. et al. Critical size defect in the goat’s os ilium. A model to evaluate bone grafts and substitutes. Clin. Orthop. Relat. Res. 1999, 231-239 (1999).
Leung, K. S. et al. Goats as an osteopenic animal model. J. Bone Miner. Res. 16, 2348–2355 (2001).
Wang, L. et al. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized β-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials 31, 9452–9461 (2010).
Liu, G. et al. Repair of goat tibial defects with bone marrow stromal cells and beta-tricalcium phosphate. J. Mater. Sci. Mater. Med. 19, 2367–2376 (2008).
Nandi, S. K., Kundu, B., Datta, S., De, D. K. & Basu, D. The repair of segmental bone defects with porous bioglass: an experimental study in goat. Res. Vet. Sci. 86, 162–173 (2009).
Qin, L., Mak, A. T., Cheng, C. W., Hung, L. K. & Chan, K. M. Histomorphological study on pattern of fluid movement in cortical bone in goats. Anat. Rec. 255, 380–387 (1999).
Newman, E., Turner, A. S. & Wark, J. D. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone 16, 277S–284S (1995).
Taylor, W. R. et al. Tibio-femoral joint contact forces in sheep. J. Biomech. 39, 791–798 (2006).
Ravaglioli, A. et al. Mineral evolution of bone. Biomaterials 17, 617–622 (1996).
Lutton, C. et al. Transplanted abdominal granulation tissue induced bone formation—an in vivo study in sheep. Connect. Tissue Res. 50, 256–262 (2009).
Jones, C. W. et al. Matrix-induced autologous chondrocyte implantation in sheep: objective assessments including confocal arthroscopy. J. Orthop. Res. 26, 292–303 (2008).
Nuss, K. M., Auer, J. A., Boos, A. & von Rechenberg, B. An animal model in sheep for biocompatibility testing of biomaterials in cancellous bones. BMC Musculoskelet. Disord. 7, 67 (2006).
Wieding, J., Lindner, T., Bergschmidt, P. & Bader, R. Biomechanical stability of novel mechanically adapted open-porous titanium scaffolds in metatarsal bone defects of sheep. Biomaterials 46, 35–47 (2015).
Viateau, V. et al. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: an ovine model. Vet. Surg. 35, 445–452 (2006).
Li, Z. et al. Repair of sheep metatarsus defects by using tissue-engineering technique. J. Huazhong Univ. Sci. Technol. Med. Sci. 25, 62–67 (2005).
Filardo, G. et al. Vegetable hierarchical structures as template for bone regeneration: new bio-ceramization process for the development of a bone scaffold applied to an experimental sheep model. J. Biomed. Mater. Res. B Appl. Biomater. https://doi.org/10.1002/jbm.b.34414 (2019).
Li, J. J. et al. A novel bone substitute with high bioactivity, strength, and porosity for repairing large and load-bearing bone defects. Adv. Healthc. Mater. 8, e1900641 (2019).
Kirby, G. T. S. et al. Microparticles for sustained growth factor delivery in the regeneration of critically-sized segmental tibial bone defects. Materials (Basel) 9, 259 (2016).
Li, J. J. et al. Efficacy of novel synthetic bone substitutes in the reconstruction of large segmental bone defects in sheep tibiae. Biomed. Mater. 11, 015016 (2016).
Berner, A. et al. Scaffold-cell bone engineering in a validated preclinical animal model: precursors vs differentiated cell source. J. Tissue Eng. Regen. Med. 11, 2081–2089 (2017).
Berner, A. et al. Delayed minimally invasive injection of allogenic bone marrow stromal cell sheets regenerates large bone defects in an ovine preclinical animal model. Stem Cells Transl. Med. 4, 503–512 (2015).
Berner, A. et al. Autologous vs. allogenic mesenchymal progenitor cells for the reconstruction of critical sized segmental tibial bone defects in aged sheep. Acta Biomater. 9, 7874–7884 (2013).
Cipitria, A. et al. BMP delivery complements the guiding effect of scaffold architecture without altering bone microstructure in critical-sized long bone defects: a multiscale analysis. Acta Biomater. 23, 282–294 (2015).
Croker, S. L., Reed, W. & Donlon, D. Comparative cortical bone thickness between the long bones of humans and five common non-human mammal taxa. Forensic Sci. Int. 260, 104 e101–104 e117 (2016).
Singh, B. Dyce, Sack and Wensing’s Textbook on Veterinary Anatomy (Elsevier, 2017).
Connolly, J. F., Guse, R., Tiedeman, J. & Dehne, R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin. Orthop. Relat. Res. 1991, 259-270 (1991).
Xerogeanes, J. W. et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann. Biomed. Eng. 26, 345–352 (1998).
Seebeck, P. et al. Gait evaluation: a tool to monitor bone healing? Clin. Biomech. (Bristol, Avon) 20, 883–891 (2005).
Ebraheim, N. A. et al. Comparison of intramedullary nail, plate, and external fixation in the treatment of distal tibia nonunions. Int. Orthop. 41, 1925–1934 (2017).
Tufekci, P. et al. Early mechanical stimulation only permits timely bone healing in sheep. J. Orthop. Res. 36, 1790–1796 (2018).
Pobloth, A. M. et al. Tubular open-porous β-tricalcium phosphate polycaprolactone scaffolds as guiding structure for segmental bone defect regeneration in a novel sheep model. J. Tissue Eng. Regen. Med. 12, 897–911 (2018).
Fernandes, M. B. et al. The effect of bone allografts combined with bone marrow stromal cells on the healing of segmental bone defects in a sheep model. BMC Vet. Res. 10, 36 (2014).
Gugala, Z. & Gogolewski, S. Healing of critical-size segmental bone defects in the sheep tibiae using bioresorbable polylactide membranes. Injury 33 (Suppl. 2), B71–B76 (2002).
Maissen, O. et al. Mechanical and radiological assessment of the influence of rhTGFbeta-3 on bone regeneration in a segmental defect in the ovine tibia: pilot study. J. Orthop. Res. 24, 1670–1678 (2006).
Herten, M. et al. Biomechanical stability and osteogenesis in a tibial bone defect treated by autologous ovine cord blood cells—a pilot study. Molecules 24, 295 (2019).
Seebeck, P. et al. Do serological tissue turnover markers represent callus formation during fracture healing? Bone 37, 669–677 (2005).
Haubruck, P. et al. Complications and risk management in the use of the reaming-irrigator-aspirator (RIA) system: RIA is a safe and reliable method in harvesting autologous bone graft. PLoS One 13, e0196051 (2018).
Ochsner, P. E., Baumgart, F. & Kohler, G. Heat-induced segmental necrosis after reaming of one humeral and two tibial fractures with a narrow medullary canal. Injury 29(Suppl. 2), B1–B10 (1998).
Christou, C., Oliver, R. A., Yu, Y. & Walsh, W. R. The Masquelet technique for membrane induction and the healing of ovine critical sized segmental defects. PLoS One 9, e114122 (2014).
Sarkar, M. R. et al. Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials 27, 1817–1823 (2006).
Tyllianakis, M. et al. Biomechanical comparison of callus over a locked intramedullary nail in various segmental bone defects in a sheep model. Med. Sci. Monit. 13, BR125–BR130 (2007).
Schneiders, W. et al. In vivo effects of modification of hydroxyapatite/collagen composites with and without chondroitin sulphate on bone remodeling in the sheep tibia. J. Orthop. Res. 27, 15–21 (2009).
Bloemers, F. W. et al. Autologous bone versus calcium-phosphate ceramics in treatment of experimental bone defects. J. Biomed. Mater. Res. B Appl. Biomater. 66, 526–531 (2003).
Hahn, J. A., Witte, T. S., Arens, D., Pearce, A. & Pearce, S. Double-plating of ovine critical sized defects of the tibia: a low morbidity model enabling continuous in vivo monitoring of bone healing. BMC Musculoskelet. Disord. 12, 214 (2011).
Niemeyer, P. et al. Xenogenic transplantation of human mesenchymal stem cells in a critical size defect of the sheep tibia for bone regeneration. Tissue Eng. Part A 16, 33–43 (2010).
Mastrogiacomo, M. et al. Reconstruction of extensive long bone defects in sheep using resorbable bioceramics based on silicon stabilized tricalcium phosphate. Tissue Eng. 12, 1261–1273 (2006).
Niemeyer, P. et al. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 31, 3572–3579 (2010).
Pobloth, A. M. et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 10, aam8828 (2018).
Giannoni, P. et al. Regeneration of large bone defects in sheep using bone marrow stromal cells. J. Tissue Eng. Regen. Med. 2, 253–262 (2008).
Chehade, M. J., Pohl, A. P., Pearcy, M. J. & Nawana, N. Clinical implications of stiffness and strength changes in fracture healing. J. Bone Jt. Surg. Br. 79, 9–12 (1997).
Augat, P. et al. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J. Orthop. Res. 21, 1011–1017 (2003).
Claes, L. et al. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J. Orthop. Res. 27, 22–27 (2009).
Steiner, M. et al. Comparison between different methods for biomechanical assessment of ex vivo fracture callus stiffness in small animal bone healing studies. PLoS One 10, e0119603 (2015).
Drakonaki, E. E., Allen, G. M. & Wilson, D. J. Ultrasound elastography for musculoskeletal applications. Br. J. Radiol. 85, 1435–1445 (2012).
Ekeland, A., Engesaeter, L. B. & Langeland, N. Mechanical properties of fractured and intact rat femora evaluated by bending, torsional and tensile tests. Acta Orthop. Scand. 52, 605–613 (1981).
Seide, K. et al. Telemetric assessment of bone healing with an instrumented internal fixator: a preliminary study. J. Bone Jt. Surg. Br. 94, 398–404 (2012).
Burny, F. et al. Concept, design and fabrication of smart orthopedic implants. Med. Eng. Phys. 22, 469–479 (2000).
Fountain, S. et al. Monitoring healing progression and characterizing the mechanical environment in preclinical models for bone tissue engineering. Tissue Eng. Part B Rev. 22, 47–57 (2016).
ASTM. Standard Guide for Pre-clinical in vivo Evaluation in Critical Size Segmental Bone Defects. (ASTM International, 2014).
Christou, C., Oliver, R. A., Rawlinson, J. & Walsh, W. R. Transdermal fentanyl and its use in ovine surgery. Res. Vet. Sci. 100, 252–256 (2015).
Cocquyt, G., Driessen, B. & Simoens, P. Variability in the eruption of the permanent incisor teeth in sheep. Vet. Rec. 157, 619–623 (2005).
Berry, S. H. Injectable anesthetics in Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones (eds K. A. Grimm et al.) (Wiley, 2015).
Goldberg, M. E. & Shaffran, N. Pain Management for Veterinary Technicians and Nurses (Wiley-Blackwell, 2014).
Quiros-Carmona, S. et al. A comparison of cardiopulmonary effects and anaesthetic requirements of two dexmedetomidine continuous rate infusions in alfaxalone-anaesthetized greyhounds. Vet. Anaesth. Analg. 44, 228–236 (2017).
Funes, F. J. et al. Anaesthetic and cardiorespiratory effects of a constant rate infusion of fentanyl in isoflurane-anaesthetized sheep. Vet. Anaesth. Analg. 42, 157–164 (2015).
Welsh, E. M., McKellar, Q. A. & Nolan, A. M. The pharmacokinetics of flunixin meglumine in the sheep. J. Vet. Pharmacol. Ther. 16, 181–188 (1993).
Blackburn, P. J., Carmichael, I. H., Walkden-Brown, S. W. & Greenslade, S. Cost of immune response to Trichostrongylus vitrinus infection in meat sheep. in Proceedings of the Australian Sheep Veterinarians, 2013 Conference (Australian Sheep Veterinarians, 2014).
Elsheikh, H. A., Osman, I. A. & Ali, B. H. Comparative pharmacokinetics of ampicillin trihydrate, gentamicin sulphate and oxytetracycline hydrochloride in Nubian goats and desert sheep. J. Vet. Pharmacol. Ther. 20, 262–266 (1997).
Reilly, J. S. Euthanasia of Animals Used for Scientific Purposes 2nd edn (Australian and New Zealand Council for the Care of Animals in Research and Teaching (ANZCCART), 2001).
Oetzel, G. R. Parturient paresis and hypocalcemia in ruminant livestock. Vet. Clin. North Am. Food Anim. Pract. 4, 351–364 (1988).
Nair, J. et al. Bioavailability of endotracheal epinephrine in an ovine model of neonatal resuscitation. Early Hum. Dev. 130, 27–32 (2019).
Lorenz, I. & Lorch, A. D-lactic acidosis in lambs. Vet. Rec. 164, 174–175 (2009).
Lankadeva, Y. R. et al. Strategies that improve renal medullary oxygenation during experimental cardiopulmonary bypass may mitigate postoperative acute kidney injury. Kidney Int. 95, 1338–1346 (2019).
Passmore, M. R. et al. Fluid resuscitation with 0.9% saline alters haemostasis in an ovine model of endotoxemic shock. Thromb. Res. 176, 39–45 (2019).
Dardenne, A. et al. Benefits of standardizing the treatment of arrhythmias in the sheep (Ovis aries) model of chronic heart failure after myocardial infarction. J. Am. Assoc. Lab. Anim. Sci. 52, 290–294 (2013).
Schauvliege, S. et al. Refined anaesthesia for implantation of engineered experimental aortic valves in the pulmonary artery using a right heart bypass in sheep. Lab. Anim. 40, 341–352 (2006).
Letzelter, J., Hill, J. B. & Hacquebord, J. An overview of skin antiseptics used in orthopaedic surgery procedures. J. Am. Acad. Orthop. Surg. 27, 599–606 (2019).
Zhou, H., Ge, J., Bai, Y., Liang, C. & Yang, L. Translation of bone wax and its substitutes: history, clinical status and future directions. J. Orthop. Transl. 17, 64–72 (2019).
Lacasta, D. et al. Vaccination schedules in small ruminant farms. Vet. Microbiol. 181, 34–46 (2015).
Leathwick, D. M. et al. Drenching adult ewes: implications of anthelmintic treatments pre- and post-lambing on the development of anthelmintic resistance. NZ Vet. J. 54, 297–304 (2006).
Araco, A., Caruso, R., Araco, F., Overton, J. & Gravante, G. Capsular contractures: a systematic review. Plast. Reconstr. Surg. 124, 1808–1819 (2009).
Pass, M. A. & Mogg, T. D. Pharmacokinetics and metabolism of amitraz in ponies and sheep. J. Vet. Pharmacol. Ther. 18, 210–215 (1995).
Grimm, K. A., Lamont, L. A., Tranquilli, W. J., Greene, S. A. & Robertson, S. A. Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones (Wiley, 2015).
Seldinger, A. I. Catheter replacement of the needle in percutaneous arteriography: a new technique. Acta Radiol. 39, 368–376 (1953).
Weingart, S. D. & Levitan, R. M. Preoxygenation and prevention of desaturation during emergency airway management. Ann. Emerg. Med. 59, 165–175 e161 (2012).
Willbold, E. & Witte, F. Histology and research at the hard tissue-implant interface using Technovit 9100 new embedding technique. Acta Biomater. 6, 4447–4455 (2010).
Donath, K. Preparation of histologic sections by a cutting-grinding technique for hard tissue and other materials not suitable to be sectioned by routine methods. in Equipment and Methodological Performance 2nd edn (EXAKT-Kulzer, 1995).
Savi, F. M., Brierly, G. I., Baldwin, J., Theodoropoulos, C. & Woodruff, M. A. Comparison of different decalcification methods using rat mandibles as a model. J. Histochem. Cytochem. 65, 705–722 (2017).
Li, J. J. et al. Effects of material-tissue interactions on bone regeneration outcomes using baghdadite implants in a large animal model. Adv. Healthc. Mater. 7, e1800218 (2018).
Paris, M. et al. Scaffold curvature-mediated novel biomineralization process originates a continuous soft tissue-to-bone interface. Acta Biomater. 60, 64–80 (2017).
Muschler, G. F., Raut, V. P., Patterson, T. E., Wenke, J. C. & Hollinger, J. O. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 16, 123–145 (2010).
Li, Y. et al. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 3, 95–104 (2015).
Mosekilde, L., Kragstrup, J. & Richards, A. Compressive strength, ash weight, and volume of vertebral trabecular bone in experimental fluorosis in pigs. Calcif. Tissue Int. 40, 318–322 (1987).
Dias, I. R. et al. Preclinical and translational studies in small ruminants (sheep and goat) as models for osteoporosis research. Curr. Osteoporos. Rep. 16, 182–197 (2018).
Moran, C. J. et al. The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 3, 1 (2016).
Fountain, S. M. Monitoring Healing Progression and Characterising the Mechanical Environment in a Preclinical Bone Defect Model. PhD thesis, Queensland University of Technology (2016).
Yong, M. R. et al. Establishment and characterization of an open mini-thoracotomy surgical approach to an ovine thoracic spine fusion model. Tissue Eng. Part C. Methods 20, 19–27 (2014).
Acknowledgements
This work was supported by the German Research Foundation (DFG; BE 4492/1-2 and HE 7074/1-1) and the Australian Research Council (ARC LP100200084, ARC IC160100026, Industrial Transformation Training Centre in Additive Biomanufacturing, ARC Future Fellowship awarded to D.W.H.). This work was also supported by funding through the Wesley Hospital Foundation, the AO Foundation and the Princess Alexandra Hospital Research Foundation. We thank the staff at the Queensland University of Technology (QUT) Medical Engineering Research Facility for veterinary assistance and administrative and technical support.
Author information
Authors and Affiliations
Contributions
D.S.S. wrote the manuscript with the assistance of S.S., F.M.S., J.R., J.A.M. and D.W.H. S.S. and J.C.R. designed and performed the experiments, and S.S. provided veterinary expertise. D.S.S., F.M.S., A.C., C.E.D., A.B., J.H., J.C.R., M. Wullschleger and J.R. performed the experiments and prepared and analyzed the data. R.S. designed, performed and analyzed the biomechanical testing. S.S., M. Wagels, M.A.W., M.A.S. and D.W.H. supervised the project, M.A.S. and M. Wagels provided clinical input into the design of the model. D.W.H. led the design of the experiments and supervised the project. All authors read and critiqued the manuscript extensively and agreed on the final version of the manuscript
Corresponding author
Ethics declarations
Competing interests
D.W.H. is a cofounder and shareholder of Osteopore International Pty Ltd, a company specializing in 3D bioresorbable implants to assist with bone healing.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Reichert, J. C. et al. Sci. Trans. Med. 4, 141ra93 (2012): https://doi.org/10.1126/scitranslmed.3003720
Berner, A. et al. Acta Biomater. 9, 7874–7884 (2013): https://doi.org/10.1016/j.actbio.2013.04.035
Berner, A. et al. J. Tissue Eng. Regen. Med. 11, 2081–2089 (2017): https://doi.org/10.1002/term.2104
Reichert, J. C. et al. Tissue Eng. B Rev. 16, 93–104 (2010): https://doi.org/10.1089/ten.TEB.2009.0455
Cipitria, A. et al. Biomaterials 34, 9960–9968 (2013): https://doi.org/10.1016/j.biomaterials.2013.09.011
Supplementary information
Rights and permissions
About this article
Cite this article
Sparks, D.S., Saifzadeh, S., Savi, F.M. et al. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat Protoc 15, 877–924 (2020). https://doi.org/10.1038/s41596-019-0271-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0271-2
This article is cited by
-
In vivo study to assess fat embolism resulting from the Reamer-Irrigator-Aspirator 2 system compared to a novel aspirator-based concept for intramedullary bone graft harvesting
Archives of Orthopaedic and Trauma Surgery (2024)
-
An in vivo study to investigate an original intramedullary bone graft harvesting technology
European Journal of Medical Research (2023)
-
Role of periosteum during healing of alveolar critical size bone defects in the mandible: a pilot study
Clinical Oral Investigations (2023)
-
A clinically relevant model of focal embolic cerebral ischemia by thrombus and thrombolysis in rhesus monkeys
Nature Protocols (2022)
-
Host responses to implants revealed by intravital microscopy
Nature Reviews Materials (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.