Abstract
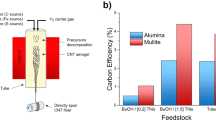
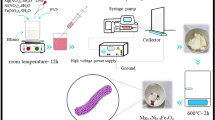
Morphology-controlled nanomaterials such as silica play a critical role in the development of technologies for use in the fields of energy, environment (water and air pollution) and health. Since the discovery of Stöber’s silica, followed by the discovery of mesoporous silica materials (MSNs) such as MCM-41 and SBA-15, a surge in the design and synthesis of nanosilica with various sizes, shapes, morphologies and textural properties (surface area, pore size and pore volume) has occurred. Dendritic fibrous nanosilica (DFNS; also known as KCC-1) is one of the recent discoveries in morphology-controlled nanomaterials. DFNS shows exceptional performance in large numbers of fields, including catalysis, gas capture, solar energy harvest, energy storage, sensors and biomedical applications. This material possesses a unique fibrous morphology, unlike the tubular porous structure of various conventional silica materials. It has a high surface area to volume ratio, with improved accessibility to the internal surface, tunable pore size and pore volume, controllable particle size and, importantly, improved stability. However, synthesis of DFNS with controllable size, textural properties and fiber density is still tricky because of several of the steps involved. This protocol provides a comprehensive step-wise description of DFNS synthesis and advice regarding how to control size, surface area, pore size, pore volume and fiber density. We also provide details of how to apply DFNS in catalysis and CO2 capture. Detailed characterization protocols for these materials using scanning electron microscopy (SEM), transmission electron microscopy (TEM), nitrogen adsorption and thermal gravimetric analysis (TGA) studies are also provided.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 813–821 (2002).
Prieto, G. et al. Hollow nano- and microstructures as catalysts. Chem. Rev. 116, 14056–14119 (2016).
Beller, M., Renken, A. & van Santen, R. A. (eds) Catalysis: From Principles to Applications (Wiley-VCH, Weinheim, Germany, 2012).
Rothenberg, G. (ed.) Catalysis: Concepts and Green Applications (Wiley-VCH, Weinheim Germany, 2008).
Polshettiwar, V. & Asefa, T. (eds) Nanocatalysis: Synthesis and Applications (Wiley, Hoboken, NJ, 2013).
Coaty, C., Zhou, H., Liu, H. & Liu, P. A scalable synthesis pathway to nanoporous metal structures. ACS Nano 12, 432–440 (2018).
Xiao, X. et al. Scalable salt-templated synthesis of two-dimensional transition metal oxides. Nat. Commun. 7, 11296 (2016).
Williamson, C. B., Nevers, D. R., Hanrath, T. & Robinson, R. D. Prodigious effects of concentration intensification on nanoparticle synthesis: a high-quality, scalable approach. J. Am. Chem. Soc. 137, 15843–15851 (2015).
Choi, S., Kim, J., Choi, N. S., Kim, M. G. & Park, S. Cost-effective scalable synthesis of mesoporous germanium particles via a redox-transmetalation reaction for high-performance energy storage devices. ACS Nano 9, 2203–2212 (2015).
Stöber, W., Fink, A. & Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 26, 62–69 (1968).
Li, S., Wan, Q., Qin, Z., Fu, Y. & Gu, Y. Understanding Stöber silica’s pore characteristics measured by gas adsorption. Langmuir 31, 824–832 (2015).
Kresge, C. T., Leonowicz, M. E., Roth, W. J., Vartuli, J. C. & Beck, J. S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359, 710–712 (1992).
Zhao, D. et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998).
Che, S. et al. A novel anionic surfactant templating route for synthesizing mesoporous silica with unique structure. Nat. Mater. 2, 801–805 (2003).
Yan, F. et al. A green and facile synthesis of ordered mesoporous nanosilica using coal fly ash. ACS Sustain. Chem. Eng. 4, 4654–4661 (2016).
Mçller, K. & Bein, T. Talented mesoporous silica nanoparticles. Chem. Mater. 29, 371–388 (2017).
Che, S. et al. Synthesis and characterization of chiral mesoporous silica. Nature 429, 281–284 (2004).
Yokoi, T. et al. Periodic arrangement of silica nanospheres assisted by amino acids. J. Am. Chem. Soc. 128, 13664–13665 (2006).
Gao, C., Sakamoto, Y., Sakamoto, K., Terasaki, O. & Che, S. Synthesis and characterization of mesoporous silica ams‐10 with bicontinuous cubic pnm symmetry. Angew. Chem. Int. Ed. 45, 4295–4298 (2006).
Bao, Y., Shi, C., Wang, T., Li, X. & Ma, J. Recent progress in hollow silica: template synthesis, morphologies and applications. Microporous Mesoporous Mater. 227, 121–136 (2016).
Polshettiwar, V., Cha, D., Zhang, X. & Basset, J. M. High surface area silica nanospheres (KCC-1) with fibrous morphology. Angew. Chem. Int. Ed. 49, 9652–9656 (2010).
Bayal, N., Singh, B., Singh, R. & Polshettiwar, V. Size and fiber density controlled synthesis of fibrous nanosilica spheres (KCC-1). Sci. Rep. 6, 24888 (2016).
Maity, A., Das, A., Sen, D., Mazumder, S. & Polshettiwar, V. Unraveling the formation mechanism of dendritic fibrous nanosilica. Langmuir 33, 13774–13782 (2017).
Maity, A. & Polshettiwar, V. Scalable and sustainable synthesis of size controlled monodisperse DFNS quantified by E-factor. ACS Appl. Nano Mater. 1, 3636–3643 (2018).
Maity, A. & Polshettiwar, V. Dendritic fibrous nanosilica for catalysis, energy harvesting, carbon dioxide mitigation, drug delivery, and sensing. ChemSusChem 10, 3866–3913 (2017).
Singh, R., Bapat, R., Qin, L. J., Feng, H. & Polshettiwar, V. Atomic layer deposited (ALD) TiO2 on fibrous nano-silica (KCC-1) for photocatalysis: nanoparticle formation and size quantization effect. ACS Catal. 6, 2770–2784 (2016).
Maity, A., Mujumdar, S. & Polshettiwar, V. Self-Assembled photonic crystals of monodisperse dendritic fibrous nanosilica for lasing: role of fiber density. ACS Appl. Mater. Interfaces. 10, 23392–23398 (2018).
Bayal, N., Singh, R. & Polshettiwar, V. Nanostructured silica-titania hybrid using fibrous nanosilica as photocatalysts. ChemSusChem 10, 2182–2191 (2017).
Kundu, S. & Polshettiwar, V. Hydrothermal crystallization of nano-titanium dioxide for enhanced photocatalytic hydrogen generation. ChemPhotoChem 2, 796–800 (2018).
Singh, B., Maity, A. & Polshettiwar, V. Synthesis of high surface area carbon nanospheres with wrinkled cages and their CO2 capture studies. Chem. Select 3, 1–6 (2018).
Singh, B. & Polshettiwar, V. Design of CO2 sorbents using functionalized fibrous nanosilica (KCC-1): insights into the effect of the silica morphology (KCC-1 vs. MCM-41). J. Mater. Chem. A 4, 7005–7019 (2016).
Huang, X. et al. Dendritic silica nanomaterials (KCC-1) with fibrous pore structure possess high DNA adsorption capacity and effectively deliver genes in vitro. Langmuir 30, 10886–10889 (2014).
Patil, U., Fihri, A., Emwas, A. H. & Polshettiwar, V. Silicon oxynitrides of KCC-1, SBA-15 and MCM-41: Unprecedented materials for CO2 capture with excellent stability and regenerability. Chem. Sci. 3, 2224–2229 (2012).
Thankamony, A. S. L. et al. Insights into the catalytic activity of nitridated fibrous silica (KCC-1) nanocatalysts from 15N and 29Si NMR enhanced by dynamic nuclear polarization. Angew. Chem. Int. Ed. 54, 2190–2193 (2015).
Polshettiwar, V. et al. “Hydro-metathesis” of olefins: a catalytic reaction using a bifunctional single-site tantalum hydride catalyst supported on fibrous silica (KCC-1) nanospheres. Angew. Chem. Int. Ed. 50, 2747–2751 (2011).
Fihri, A. et al. Fibrous nano-silica supported ruthenium (KCC-1/Ru): a sustainable catalyst for the hydrogenolysis of alkanes with good catalytic activity and lifetime. ACS Catal. 2, 1425–1431 (2012).
Dhiman, M., Chalke, B. & Polshettiwar, V. Efficient synthesis of monodisperse metal (Rh, Ru, Pd) nanoparticles supported on fibrous nanosilica (KCC-1) for catalysis. ACS Sustain. Chem. Eng. 3, 3224–3230 (2015).
Dhiman, M., Chalke, B. & Polshettiwar, V. Organosilane oxidation with a half million turnover number using fibrous nanosilica supported ultrasmall nanoparticles and pseudo-single atoms of gold. J. Mater. Chem. A 5, 1935–1940 (2017).
Dhiman, M. & Polshettiwar, V. Ultrasmall nanoparticles and pseudo single atoms of platinum supported on fibrous nanosilica (KCC-1/Pt): engineering selectivity of hydrogenation reactions. J. Mat. Chem. A 4, 12416–12424 (2016).
Fihri, A., Cha, D., Bouhrara, M., Almana, N. & Polshettiwar, V. Fibrous nano-silica (KCC-1)-supported palladium satalyst: Suzuki coupling reactions under sustainable conditions. ChemSusChem 5, 85–89 (2012).
Jung, D., Kim, Y.-J. & Lee, J.-K. Novel strategy for maintenance of catalytic activity using wrinkled silica nanoparticle support in Fischer–Tropsch synthesis. Bull. Korean Chem. Soc. 37, 386–389 (2016).
Afzal, S., Quan, X., Chen, S., Wang, J. & Muhammad, D. Synthesis of manganese incorporated hierarchical mesoporous silicananosphere with fibrous morphology by facile one-pot approach for efficient catalytic ozonation. J. Hazard. Mater. 318, 308–318 (2016).
Tian, Y. et al. Fibrous porous silica microspheres decorated with Mn3O4 for effective removal of methyl orange from aqueous solution. RSC Adv. 5, 106068–106076 (2015).
Firmansyah, M. L. et al. Synthesis and characterization of fibrous silica ZSM‐5 for cumene hydrocracking. Catal. Sci. Technol. 6, 5178–5182 (2016).
Yang, Y. et al. Amphiphilic titanosilicates as pickering interfacial catalysts for liquid-phase oxidation reactions. J. Phys. Chem. C 119, 25377–25384 (2015).
Zhang, J., Zhang, M., Yang, C. & Wang, X. Nanospherical carbon nitride frameworks with sharp edges accelerating charge collection and separation at a soft photocatalytic interface. Adv. Mater. 26, 4121–4126 (2014).
Ryu, J., Yun, J., Lee, J., Lee, K. & Jang, J. Hierarchical mesoporous silica nanoparticles as superb light scattering materials. Chem. Commun. 52, 2165–2168 (2016).
Chen, R. et al. Polypyrrole confined in dendrimer-like silica nanoparticles for combined photothermal and chemotherapy of cancer. RSC Adv. 6, 38931–38942 (2016).
Du, X. et al. Broadband antireflective superhydrophobic selfcleaning coatings based on novel dendritic porous particles. RSC Adv. 6, 7864–7871 (2016).
Zhang, S. et al. Facile fabrication of dendritic mesoporous SiO2@CdTe@SiO2 fluorescent nanoparticles for bioimaging. Part. Part. Syst. Charact. 33, 261–270 (2016).
Choi, Y., Kwak, H. & Hong, S. Quantification of arsenic(III) in aqueous media using a novel hybrid platform comprised of radially porous silica particles and a gold thin film. Anal. Methods 6, 7054–7061 (2014).
Qu, Q., Min, Y., Zhang, L., Xu, Q. & Yin, Y. Silica microspheres with fibrous shells: synthesis and application in HPLC. Anal. Chem. 87, 9631–9638 (2015).
Guo, D. et al. Hydrangea-like multi-scale carbon hollow submicron spheres with hierarchical pores for high performance supercapacitor electrodes. Electrochim. Acta 176, 207–214 (2015).
Wang, Y. et al. Dendritic silica particles with well-dispersed ag nanoparticles for robust antireflective and antibacterial nanocoatings on polymeric glass. ACS Sustain. Chem. Eng. 6, 14071–14081 (2018).
Acknowledgements
This work was supported by the Department of Atomic Energy (DAE), Government of India. We would like to also thank Indo-France CEFIPRA and SHELL Industries for funding that partially supported this work. We acknowledge the EM, XRD and NMR Facility of TIFR, Mumbai.
Author information
Authors and Affiliations
Contributions
V.P. proposed the research direction and guided the project. A.M. and V.P. developed the protocol. A.M. and R.B. performed the experiments. A.M. and V.P. drafted the manuscript. All authors contributed to the manuscript. We thank S. Rawool and M. Dhiman for their support during the preparation of this protocol.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Protocols thanks Ling Chao and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Maity, A. & Polshettiwar, V. ChemSusChem 10, 3866−3913 (2017): https://onlinelibrary.wiley.com/doi/full/10.1002/cssc.201701076
Polshettiwar, V., Cha, D., Zhang, X. & Basset, J. M. Angew. Chem. Int. Ed. 49, 9652−9656 (2010): https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201003451
Key data used in this protocol
Maity, A. & Polshettiwar, V. ACS Appl. Nano Mater. 1, 3636–3643 (2018): https://pubs.acs.org/doi/10.1021/acsanm.8b00761
Maity, A., Das, A., Sen, D., Mazumder, S. & Polshettiwar, V. Langmuir 33, 13774−13782 (2017): https://pubs.acs.org/doi/10.1021/acs.langmuir.7b02996
Singh, B. & Polshettiwar, V. J. Mater. Chem. A 4, 7005–7019 (2016): https://pubs.rsc.org/is/content/articlehtml/2016/ta/c6ta01348a
Fihri, A., Cha, D., Bouhrara, M., Almana, N. & Polshettiwar, V. ChemSusChem 5, 85–89 (2012): https://onlinelibrary.wiley.com/doi/full/10.1002/cssc.201100379
Maity, A. & Polshettiwar, V. ChemSusChem 10, 3866−3913 (2017): https://onlinelibrary.wiley.com/doi/full/10.1002/cssc.201701076
Polshettiwar, V., Cha, D., Zhang, X. & Basset J. M. Angew. Chem. Int. Ed. 49, 9652−9656 (2010): https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201003451
Supplementary information
Rights and permissions
About this article
Cite this article
Maity, A., Belgamwar, R. & Polshettiwar, V. Facile synthesis to tune size, textural properties and fiber density of dendritic fibrous nanosilica for applications in catalysis and CO2 capture. Nat Protoc 14, 2177–2204 (2019). https://doi.org/10.1038/s41596-019-0177-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0177-z
This article is cited by
-
Pt-doped Ru nanoparticles loaded on ‘black gold’ plasmonic nanoreactors as air stable reduction catalysts
Nature Communications (2024)
-
Facile synthesis and fine morphological tuning of dendritic mesoporous silica & titania nanospheres
Journal of Porous Materials (2024)
-
PdMoSb trimetallene as high-performance alcohol oxidation electrocatalyst
Nano Research (2023)
-
Synthesis of Cyclic Carbonate from Carbon Dioxide and Epoxides Using Bicobalt Complexes Absorbed on DFNS
Catalysis Letters (2023)
-
A novel pH- and glutathione-responsive drug delivery system based on in situ growth of MOF199 on mesoporous organic silica nanoparticles targeting the hepatocellular carcinoma niche
Cancer Nanotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.