Abstract
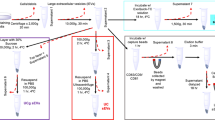
We describe the protocol development and optimization of asymmetric-flow field-flow fractionation (AF4) technology for separating and characterizing extracellular nanoparticles (ENPs), particularly small extracellular vesicles (sEVs), known as exosomes, and even smaller novel nanoparticles, known as exomeres. This technique fractionates ENPs on the basis of hydrodynamic size and demonstrates a unique capability to separate nanoparticles with sizes ranging from a few nanometers to an undefined level of micrometers. ENPs are resolved by two perpendicular flows—channel flow and cross-flow—in a thin, flat channel with a semi-permissive bottom wall membrane. The AF4 separation method offers several advantages over other isolation methods for ENP analysis, including being label-free, gentle, rapid (<1 h) and highly reproducible, as well as providing efficient recovery of analytes. Most importantly, in contrast to other available techniques, AF4 can separate ENPs at high resolution (1 nm) and provide a large dynamic range of size-based separation. In conjunction with real-time monitors, such as UV absorbance and dynamic light scattering (DLS), and an array of post-separation characterizations, AF4 facilitates the successful separation of distinct subsets of exosomes and the identification of exomeres. Although the whole procedure of cell culture and ENP isolation from the conditioned medium by ultracentrifugation (UC) can take ~3 d, the AF4 fractionation step takes only 1 h. Users of this technology will require expertise in the working principle of AF4 to operate and customize protocol applications. AF4 can contribute to the development of high-quality, exosome- and exomere-based molecular diagnostics and therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All Astra 6 data files used for producing the plots presented in figures have been deposited at https://figshare.com/s/6f22aede51fb279a3f81.
References
Théry, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002).
EL Andaloussi, S., Mäger, I., Breakefield, X. O. & Wood, M. J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357 (2013).
Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013).
Di Vizio, D. et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 69, 5601–5609 (2009).
Morello, M. et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle 12, 3526–3536 (2013).
Minciacchi, V. R. et al. MYC mediates large oncosome-induced fibroblast reprogramming in prostate cancer. Cancer Res. 77, 2306–2317 (2017).
Balaj, L. et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180 (2011).
Choi, D. S., Kim, D. K., Kim, Y. K. & Gho, Y. S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 13, 1554–1571 (2013).
Thakur, B. K. et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 24, 766–769 (2014).
Tetta, C., Ghigo, E., Silengo, L., Deregibus, M. C. & Camussi, G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 44, 11–19 (2013).
Zhang, H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332–343 (2018).
Aalberts, M. et al. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol. Reprod. 86, 82 (2012).
Caby, M. P., Lankar, D., Vincendeau-Scherrer, C., Raposo, G. & Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 17, 879–887 (2005).
Huebner, A. R. et al. Exosomes in urine biomarker discovery. Adv. Exp. Med. Biol. 845, 43–58 (2015).
Ogawa, Y. et al. Proteomic analysis of two types of exosomes in human whole saliva. Biol. Pharm. Bull. 34, 13–23 (2011).
Admyre, C. et al. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179, 1969–1978 (2007).
Navabi, H. et al. Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol. Dis. 35, 149–152 (2005).
Street, J. M. et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 10, 5 (2012).
Théry, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter 3, Unit 3.22 (2006).
Merchant, M. L. et al. Microfiltration isolation of human urinary exosomes for characterization by MS. Proteomics Clin. Appl. 4, 84–96 (2010).
Lässer, C., Eldh, M. & Lötvall, J. Isolation and characterization of RNA-containing exosomes. J. Vis. Exp. 2012, e3037 (2012).
Chen, C. et al. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 10, 505–511 (2010).
Jørgensen, M. et al. Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J. Extracell. Vesicles 2, 20920 (2013).
Tauro, B. J. et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56, 293–304 (2012).
Fraunhofer, W. & Winter, G. The use of asymmetrical flow field-flow fractionation in pharmaceutics and biopharmaceutics. Eur. J. Pharm. Biopharm. 58, 369–383 (2004).
Yohannes, G., Jussila, M., Hartonen, K. & Riekkola, M. L. Asymmetrical flow field-flow fractionation technique for separation and characterization of biopolymers and bioparticles. J. Chromatogr. A 1218, 4104–4116 (2011).
Giddings, C. J. A new concept based on a coupling of concentration and flow nonuniformities. Sep. Sci. 1, 123–125 (1966).
Berg, H. C., Purcell, E. M. & Stewart, W. W. A method for separating according to mass a mixture of macromolecules or small particles suspended in a fluid, 3. Experiments in a centrifugal fluid. Proc. Natl. Acad. Sci. USA 58, 1818–1828 (1967).
Yang, F. J. F., Myers, M. N. & Giddings, J. C. Programmed sedimentation field-flow fractionation. Anal. Chem. 46, 1924–1930 (1974).
Giddings, J. C., Martin, M. & Myers, M. N. High-speed polymer separations by thermal field-flow fractionation. J. Chromatogr. A 158, 419–435 (1978).
Caldwell, K. D. & Gao, Y. S. Electrical field-flow fractionation in particle separation. 1. Monodisperse standards. Anal. Chem. 65, 1764–1772 (1993).
Giddings, J. C., Yang, F. J. & Myers, M. N. Flow-field-flow fractionation: a versatile new separation method. Science 193, 1244–1245 (1976).
Granger, J., Dodds, J., Leclerc, D. & Midoux, N. Flow and diffusion of particles in a channel with one porous wall: polarization chromatography. Chem. Eng. Sci. 41, 3119–3128 (1986).
Wahlund, K. G. & Giddings, J. C. Properties of an asymmetrical flow field-flow fractionation channel having one permeable wall. Anal. Chem. 59, 1332–1339 (1987).
Litzén, A. & Wahlund, K. G. Improved separation speed and efficiency for proteins, nucleic acids and viruses in asymmetrical flow field flow fractionation. J. Chromatogr. A 476, 413–421 (1989).
Wahlund, K. G. & Litzén, A. Application of an asymmetrical flow field-flow fractionation channel to the separation and characterization of proteins, plasmids, plasmid fragments, polysaccharides and unicellular algae. J. Chromatogr. A 461, 73–87 (1989).
Yohannes, G. et al. Miniaturization of asymmetrical flow field-flow fractionation and application to studies on lipoprotein aggregation and fusion. Anal. Biochem. 354, 255–265 (2006).
Wittgren, B. & Wahlund, K.-G. Fast molecular mass and size characterization of polysaccharides using asymmetrical flow field-flow fractionation-multiangle light scattering. J. Chromatogr. A 760, 205–218 (1997).
Wei, Z. et al. Biophysical characterization of influenza virus subpopulations using field flow fractionation and multiangle light scattering: correlation of particle counts, size distribution and infectivity. J. Virol. Methods 144, 122–132 (2007).
Chuan, Y. P., Fan, Y. Y., Lua, L. & Middelberg, A. P. Quantitative analysis of virus-like particle size and distribution by field-flow fractionation. Biotechnol. Bioeng. 99, 1425–1433 (2008).
Oh, S. et al. Miniaturized asymmetrical flow field-flow fractionation: application to biological vesicles. J. Sep. Sci. 30, 1082–1087 (2007).
Sitar, S. et al. Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation. Anal. Chem. 87, 9225–9233 (2015).
Petersen, K. E. et al. A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal. Bioanal. Chem. 406, 7855–7866 (2014).
Ashby, J. et al. Distribution profiling of circulating microRNAs in serum. Anal. Chem. 86, 9343–9349 (2014).
Cölfen, H. & Antonietti, M. Field-flow fractionation techniques for polymer and colloid analysis. Adv. Polym. Sci. 150, 67–187 (2000).
Litzen, A. & Wahlund, C. G. Zone broadening and dilution in rectangular and trapezoidal asymmetrical flow field-flow fractionation channels. Anal. Chem. 63, 1001–1007 (1991).
Batrakova, E. V. & Kim, M. S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 219, 396–405 (2015).
Jeppesen, D. K. et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J Extracell. Vesicles 3, 25011 (2014).
Cvjetkovic, A., Lötvall, J. & Lässer, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles 3, 23111 (2014).
Willms, E. et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 6, 22519 (2016).
Bobrie, A., Colombo, M., Krumeich, S., Raposo, G. & Théry, C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J. Extracell. Vesicles 1, 18397 (2012).
Mol, E. A., Goumans, M. J., Doevendans, P. A., Sluijter, J. P. G. & Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 13, 2061–2065 (2017).
Nordin, J. Z. et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine 11, 879–883 (2015).
Böing, A. N. et al. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 3 (2014).
Willis, G. R., Kourembanas, S. & Mitsialis, S. A. Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front. Cardiovasc. Med. 4, 63 (2017).
Xu, R., Greening, D. W., Rai, A., Ji, H. & Simpson, R. J. Highly-purified exosomes and shed microvesicles isolated from the human colon cancer cell line LIM1863 by sequential centrifugal ultrafiltration are biochemically and functionally distinct. Methods 87, 11–25 (2015).
Xu, R., Simpson, R. J. & Greening, D. W. A protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. Methods Mol. Biol. 1545, 91–116 (2017).
Ko, J. et al. miRNA profiling of magnetic nanopore-isolated extracellular vesicles for the diagnosis of pancreatic cancer. Cancer Res. 78, 3688–3697 (2018).
Wyatt Technology. DYNAMICS User’s Guide. Version 7.0 (M1400 Rev. I) Appendix A-2 (Wyatt Technology, Santa Barbara, CA, 2010).
Acknowledgements
We are grateful for the great AF4 technical support from Wyatt Technology. We thank our colleagues I. Matei and C. Kenific for comments on this protocol. Our study was supported by the National Cancer Institute (U01-CA169538, D.L.), the National Institutes of Health (R01-CA169416, D.L.; R01-CA218513, D.L. and H.Z.), the United States Department of Defense (W81XWH-13-1-0249, D.L.; W81XWH-13-1-0427, D.L.), the Sohn Conference Foundation (H.Z.), the Children’s Cancer and Blood Foundation (D.L.), the Manning Foundation (D.L.), the Hartwell Foundation (D.L.), the Nancy C. and Daniel P. Paduano Foundation (D.L.), The Starr Cancer Consortium (D.L. and H.Z.), the Pediatric Oncology Experimental Therapeutic Investigator Consortium (POETIC, D.L.), the James Paduano Foundation (D.L.), the National Institutes of Health/WCM CTSC (NIH/NCATS UL1TR00457 (H.Z.); NIH/NCATS UL1TR002384 (D.L. and H.Z.)), Thompson Family Foundation (D.L.), and Malcolm Hewitt Wiener Foundation (D.L.).
Author information
Authors and Affiliations
Contributions
D.L. and H.Z. designed and technically developed the protocol. H.Z. performed the experiments. D.L. and H.Z. analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Protocols thanks Pieter Vader and other (anonymous) reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related link
Key reference using this protocol
Zhang, H. et al. Nat. Cell Biol. 20, 332–343 (2018): https://doi.org/10.1038/s41556-018-0040-4
Integrated supplementary information
Supplementary Figure 1 Estimation of the yield recovered for exomere, Exo-S and Exo-L derived from B16-F10 and AsPC-1.
Shown in a and b are the yield recovered for exomere, Exo-S and Exo-L derived from B16-F10 and AsPC-1 (100 μg of sEV input for AF4), respectively. c and d show the average concentration of unconcentrated fraction post AF4 fractionation for exomere, Exo-S and Exo-L derived from B16-F10 and AsPC-1, respectively. Data are presented as mean ± s.e.m. and three independent experiments for each cell type are analyzed.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1
Rights and permissions
About this article
Cite this article
Zhang, H., Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc 14, 1027–1053 (2019). https://doi.org/10.1038/s41596-019-0126-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0126-x
This article is cited by
-
High-yield and rapid isolation of extracellular vesicles by flocculation via orbital acoustic trapping: FLOAT
Microsystems & Nanoengineering (2024)
-
Extracellular vesicles: powerful candidates in nano-drug delivery systems
Drug Delivery and Translational Research (2024)
-
miR-92a-1-5p enriched prostate cancer extracellular vesicles regulate osteoclast function via MAPK1 and FoxO1
Journal of Experimental & Clinical Cancer Research (2023)
-
Extracellular vesicles: a rising star for therapeutics and drug delivery
Journal of Nanobiotechnology (2023)
-
Modeling and optimization of parallelized immunomagnetic nanopore sorting for surface marker specific isolation of extracellular vesicles from complex media
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.