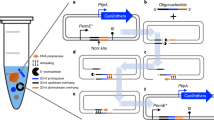
Abstract
The study of fungal pathogens is of immediate importance, yet progress is hindered by the technical challenges of genetic manipulation. For Candida species, their inability to maintain plasmids, unusual codon usage, and inefficient homologous recombination are among the obstacles limiting efficient genetic manipulation. New advances in genomic biotechnologies—particularly CRISPR-based tools—have revolutionized genome editing for many fungal species. Here, we present a protocol for CRISPR–Cas9-based manipulation in Candida albicans using a modified gene-drive-based strategy that takes ~1 month to complete. We detail the generation of Candida-optimized Cas9-based plasmids for gene deletion, an efficient transformation protocol using C. albicans haploids, and an optimized mating strategy to generate homozygous single- and double-gene diploid mutants. We further describe protocols for quantifying cell growth and analysis pipelines to calculate fitness and genetic interaction scores for genetic mutants. This protocol overcomes previous limitations associated with genetic manipulation in C. albicans and advances researchers’ ability to perform genetic analysis in this pathogen; the protocol also has broad applicability to other mating-competent microorganisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
11 April 2019
The version of this paper originally published contained reference errors. The sentence “To dissect complex genetic interactions in C. albicans, a CRISPR–Cas9-based Gene Drive Array (GDA) was developed” incorrectly cited ref. 13, and should have cited ref. 14. In addition, the reference included as ref. 13 in the original paper was incorrect, and should have been the following: Shapiro, R. S., Chavez, A. & Collins, J. J. CRISPR-based genomic tools for the manipulation of genetically intractable microorganisms. Nat. Rev. Microbiol. 16, 333–339 (2018). This reference should have been cited after the sentence “Recent innovations in CRISPR–Cas9-based genome editing have facilitated such genetic interaction analyses.” The original reference 13 (Gerami-Nejad, M., Zacchi, L. F., McClellan, M., Matter, K. & Berman, J. Shuttle vectors for facile gap repair cloning and integration into a neutral locus in Candida albicans. Microbiology 159, 565–579 (2013)) should have been cited later in the paper, and is now in the reference list as ref. 27. As a result, original references 27–33 have been renumbered in the reference list and in the text. These changes have been made in the PDF and HTML versions of the protocol.
References
Denning, D. D. Global fungal disease burden. Eur. J. Clin. Microbiol. Infect. Dis. 36, 923–1062 (2017).
Bongomin, F., Gago, S., Oladele, R. & Denning, D. Global and multi-national prevalence of fungal diseases—estimate precision. J. Fungi 3, 57 (2017).
Geddes-McAlister, J. & Shapiro, R. S. New pathogens, new tricks: emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Ann. N. Y. Acad. Sci. 1435, 57–78 (2018).
Brown, G. D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13 (2012).
Martin, G. S., Mannino, D. M., Eaton, S. & Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 (2003).
Pfaller, M. A. et al. Global trends in the antifungal susceptibility of Cryptococcus neoformans (1990 to 2004). J. Clin. Microbiol. 43, 2163–2167 (2005).
Pfaller, M. A. & Diekema, D. J. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20, 133–163 (2007).
McCarty, T. P. & Pappas, P. G. Invasive candidiasis. Infect. Dis. Clin. North Am. 30, 103–124 (2016).
Shapiro, R. S., Robbins, N. & Cowen, L. E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 75, 213–267 (2011).
Baryshnikova, A., Costanzo, M., Myers, C. L., Andrews, B. & Boone, C. Genetic interaction networks: toward an understanding of heritability. Annu. Rev. Genomics Hum. Genet. 14, 111–133 (2013).
Dixon, S. J., Costanzo, M., Baryshnikova, A., Andrews, B. & Boone, C. Systematic mapping of genetic interaction networks. Annu. Rev. Genet. 43, 601–625 (2009).
Boucher, B. & Jenna, S. Genetic interaction networks: better understand to better predict. Front. Genet. 4, 290 (2013).
Shapiro, R. S., Chavez, A. & Collins, J. J. CRISPR-based genomic tools for the manipulation of genetically intractable microorganisms. Nat. Rev. Microbiol. 16, 333–339 (2018).
Shapiro, R. S. et al. A CRISPR–Cas9-based gene drive platform for genetic interaction analysis in Candida albicans. Nat. Microbiol. 3, 73–82 (2018).
Costanzo, M. et al. The genetic landscape of a cell. Science 327, 425–431 (2010).
Costanzo, M. et al. A global genetic interaction network maps a wiring diagram of cellular function. Science 353, aaf1420-1–aaf1420-14 (2016).
Tong, A. H. et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294, 2364–2368 (2001).
Tong, A. H. Y. et al. Global mapping of the yeast genetic interaction network. Science 303, 808–813 (2004).
van Haaften, G., Vastenhouw, N. L., Nollen, E. A. A., Plasterk, R. H. A. & Tijsterman, M. Gene interactions in the DNA damage-response pathway identified by genome-wide RNA-interference analysis of synthetic lethality. Proc. Natl Acad. Sci. USA 101, 12992–12996 (2004).
Laufer, C., Fischer, B., Billmann, M., Huber, W. & Boutros, M. Mapping genetic interactions in human cancer cells with RNAi and multiparametric phenotyping. Nat. Methods 10, 427–431 (2013).
Homann, O. R., Dea, J., Noble, S. M. & Johnson, A. D. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 5, e1000783 (2009).
Noble, S. M., French, S., Kohn, L. A., Chen, V. & Johnson, A. D. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 42, 590–598 (2010).
Roemer, T. et al. Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol. Microbiol. 50, 167–181 (2003).
Ryan, O. et al. Global gene deletion analysis exploring yeast filamentous growth. Science 337, 1353–1356 (2012).
O’Meara, T. R. et al. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat. Commun. 6, 6741 (2015).
Lee, J. A., Robbins, N., Xie, J. L., Ketela, T. & Cowen, L. E. Functional genomic analysis of Candida albicans adherence reveals a key role for the Arp2/3 complex in cell wall remodelling and biofilm formation. PLoS Genet. 12, e1006452 (2016).
Gerami-Nejad, M., Zacchi, L. F., McClellan, M., Matter, K. & Berman, J. Shuttle vectors for facile gap repair cloning and integration into a neutral locus in Candida albicans. Microbiology 159, 565–579 (2013).
DiCarlo, J. E., Chavez, A., Dietz, S. L., Esvelt, K. M. & Church, G. M. Safeguarding CRISPR–Cas9 gene drives in yeast. Nat. Biotechnol. 33, 1250–1255 (2015).
Hickman, M. A. et al. The ‘obligate diploid’ Candida albicans forms mating-competent haploids. Nature 494, 55–59 (2013).
Miller, M. G. & Johnson, A. D. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110, 293–302 (2002).
Bennett, R. J. & Johnson, A. D. Mating in Candida albicans and the search for a sexual cycle. Annu. Rev. Microbiol. 59, 233–255 (2005).
Cannon, R. D. et al. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 22, 291–321 (2009).
Thompson, D. A. et al. Evolutionary principles of modular gene regulation in yeasts. Elife 2, e00603 (2013).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Acknowledgements
This work was supported by an NSERC Discovery Grant, an NSERC Discovery Accelerator Supplement, and a Banting Research Foundation Discovery Award to R.S.S. A.C. was supported by the Burroughs Wellcome Fund Career Award for Medical Scientists.
Author information
Authors and Affiliations
Contributions
The protocol was conceived and developed by C.B.M.P., A.C., and R.S.S. The manuscript was written by V.H. and R.S.S., with contributions from C.B.M.P. and A.C. Experiments were performed by V.H. All authors contributed to editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related link
Key reference using this protocol
Shapiro, R. S. et al. Nat. Microbiol. 3, 73–82 (2018): https://doi.org/10.1038/s41564-017-0043-0
Rights and permissions
About this article
Cite this article
Halder, V., Porter, C.B.M., Chavez, A. et al. Design, execution, and analysis of CRISPR–Cas9-based deletions and genetic interaction networks in the fungal pathogen Candida albicans. Nat Protoc 14, 955–975 (2019). https://doi.org/10.1038/s41596-018-0122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0122-6
This article is cited by
-
Updated immunomodulatory roles of gut flora and microRNAs in inflammatory bowel diseases
Clinical and Experimental Medicine (2022)
-
Ten decadal advances in fungal biology leading towards human well-being
Fungal Diversity (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.