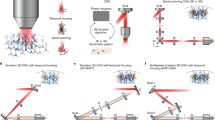
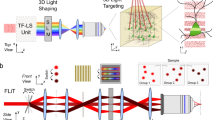
Abstract
Optogenetic tools provide users the ability to photocontrol the activity of cells. Commonly, activation is achieved by expression of proteins from photosynthetic organisms, for example, microbial opsins (e.g., ChR2). Alternatively, a sister approach, synthetic optogenetics, enables photocontrol over proteins of mammalian origin by use of photoswitches, visible light (typically), and genetic modification. Thus, synthetic optogenetics facilitates interrogation of native neuronal signaling mechanisms. However, the poor tissue penetration of visible wavelengths impedes the use of the technique in tissue, as two-photon excitation (2PE) is typically required to access the near-infrared window. Here, we describe an alternative technique that uses 2PE-compatible photoswitches (section 1) for photoactivation of genetically modified glutamate receptors (section 2). Furthermore, for fast, multi-region photoactivation, we describe the use of 2P-digital holography (2P-DH) (section 3). We detail how to combine 2P-DH and synthetic optogenetics with electrophysiology, or with red fluorescence Ca2+ recordings, for all-optical neural interrogation. The time required to complete the methods, aside from obtaining the necessary reagents and illumination equipment, is ~3 weeks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The authors declare that most data supporting the findings of this study are available within the paper; however, data are also available from the corresponding author upon request.
References
Ji, N., Shroff, H., Zhong, H. & Betzig, E. Advances in the speed and resolution of light microscopy. Curr. Opin. Neurobiol. 18, 605–616 (2008).
Ramon, Y. C. S. Structure and connections of neurons. Bull. Los Angeles Neurol. Soc. 17, 5–46 (1952).
Rodriguez, E. A. et al. The growing and glowing toolbox of fluorescent and photoactive proteins. Trends Biochem. Sci. 42, 111–129 (2017).
Nagel, G. et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296, 2395–2398 (2002).
Deisseroth, K. et al. Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci. 26, 10380–10386 (2006).
Kaufman, H., Vratsanos, S. M. & Erlanger, B. F. Photoregulation of an enzymic process by means of a light-sensitive ligand. Science 162, 1487–1489 (1968).
Szobota, S. & Isacoff, E. Y. Optical control of neuronal activity. Annu. Rev. Biophys. 39, 329–348 (2010).
Kramer, R. H., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 16, 816–823 (2013).
Reiner, A. & Isacoff, E. Y. Photoswitching of cell surface receptors using tethered ligands. Methods Mol. Biol. 1148, 45–68 (2014).
Kienzler, M. A. & Isacoff, E. Y. Precise modulation of neuronal activity with synthetic photoswitchable ligands. Curr. Opin. Neurobiol. 45, 202–209 (2017).
Berlin, S. & Isacoff, E. Y. Synapses in the spotlight with synthetic optogenetics. EMBO Rep. 18, 677–692 (2017).
Berlin, S. & Isacoff, E. Y. in Biochemical Approaches for Glutamatergic Neurotransmission (eds. Parrot, S. & Denoroy, L.) 293–325 (Springer, New York, 2018).
Mourot, A., Herold, C., Kienzler, M. A. & Kramer, R. H. Understanding and improving photo-control of ion channels in nociceptors with azobenzene photo-switches. Br. J. Pharmacol. 175, 2296–2311 (2018).
Gorostiza, P. & Isacoff, E. Y. Optical switches for remote and noninvasive control of cell signaling. Science 322, 395–399 (2008).
Izquierdo-Serra, M. et al. Optical control of endogenous receptors and cellular excitability using targeted covalent photoswitches. Nat. Commun. 7, 12221 (2016).
Mourot, A. et al. Rapid optical control of nociception with an ion-channel photoswitch. Nat. Methods 9, 396–402 (2012).
Carroll, E. C. et al. Two-photon brightness of azobenzene photoswitches designed for glutamate receptor optogenetics. Proc. Natl. Acad. Sci. USA 112, E776–E785 (2015).
Kienzler, M. A. et al. A red-shifted, fast-relaxing azobenzene photoswitch for visible light control of an ionotropic glutamate receptor. J. Am. Chem. Soc. 135, 17683–17686 (2013).
Laprell, L. et al. Optical control of NMDA receptors with a diffusible photoswitch. Nat. Commun. 6, 8076 (2015).
Passlick, S., Richers, M. T. & Ellis-Davies, G. C. R. Thermodynamically stable, photoreversible pharmacology in neurons with one- and two-photon excitation. Angew. Chem. Int. Ed. Engl. 57, 12554–12557 (2018).
Konrad, D. B., Frank, J. A. & Trauner, D. Synthesis of redshifted azobenzene photoswitches by late-stage functionalization. Chemistry 22, 4364–4368 (2016).
Izquierdo-Serra, M. et al. Two-photon neuronal and astrocytic stimulation with azobenzene-based photoswitches. J. Am. Chem. Soc. 136, 8693–8701 (2014).
Hull, K., Morstein, J. & Trauner, D. In vivo photopharmacology. Chem. Rev. 118, 10710–10747 (2018).
Dong, M., Babalhavaeji, A., Samanta, S., Beharry, A. A. & Woolley, G. A. Red-shifting azobenzene photoswitches for in vivo use. Acc. Chem. Res. 48, 2662–2670 (2015).
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Bockmann, M., Doltsinis, N. L. & Marx, D. Nonadiabatic hybrid quantum and molecular mechanic simulations of azobenzene photoswitching in bulk liquid environment. J. Phys. Chem. A 114, 745–754 (2010).
Berlin, S. et al. A family of photoswitchable NMDA receptors. Elife 5, e12040 (2016).
Spicer, C. D. & Davis, B. G. Selective chemical protein modification. Nat. Commun. 5, 4740 (2014).
Leippe, P., Koehler Leman, J. & Trauner, D. Specificity and speed: tethered photopharmacology. Biochemistry 56, 5214–5220 (2017).
Durand-de Cuttoli, R. et al. Manipulating midbrain dopamine neurons and reward-related behaviors with light-controllable nicotinic acetylcholine receptors. Elife 7, e37487 (2018).
Lin, W. C. et al. A comprehensive optogenetic pharmacology toolkit for in vivo control of GABA(A) receptors and synaptic inhibition. Neuron 88, 879–891 (2015).
Reiner, A. & Isacoff, E. Y. Tethered ligands reveal glutamate receptor desensitization depends on subunit occupancy. Nat. Chem. Biol. 10, 273–280 (2014).
Fodje, M. N. & Al-Karadaghi, S. Occurrence, conformational features and amino acid propensities for the pi-helix. Protein Eng. 15, 353–358 (2002).
Wyart, C. et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 461, 407–410 (2009).
Volgraf, M. et al. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2, 47–52 (2006).
Levitz, J. et al. Optical control of metabotropic glutamate receptors. Nat. Neurosci. 16, 507–516 (2013).
Numano, R. et al. Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR. Proc. Natl. Acad. Sci. USA 106, 6814–6819 (2009).
Rullo, A. et al. Long wavelength optical control of glutamate receptor ion channels using a tetra-ortho-substituted azobenzene derivative. Chem. Commun. 50, 14613–14615 (2014).
Burnashev, N., Zhou, Z., Neher, E. & Sakmann, B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J. Physiol. 485, 403–418 (1995).
Schneggenburger, R., Zhou, Z., Konnerth, A. & Neher, E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron 11, 133–143 (1993).
Zucker, R. S. Calcium- and activity-dependent synaptic plasticity. Curr. Opin. Neurobiol. 9, 305–313 (1999).
Li, D., Herault, K., Isacoff, E. Y., Oheim, M. & Ropert, N. Optogenetic activation of LiGluR-expressing astrocytes evokes anion channel-mediated glutamate release. J. Physiol. 590, 855–873 (2012).
Szobota, S. et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron 54, 535–545 (2007).
Niswender, C. M. & Conn, P. J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322 (2010).
Kunishima, N. et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407, 971–977 (2000).
Oron, D., Papagiakoumou, E., Anselmi, F. & Emiliani, V. Two-photon optogenetics. Prog. Brain Res. 196, 119–143 (2012).
Chen, I. W., Papagiakoumou, E. & Emiliani, V. Towards circuit optogenetics. Curr. Opin. Neurobiol. 50, 179–189 (2018).
Yang, W. & Yuste, R. Holographic imaging and photostimulation of neural activity. Curr. Opin. Neurobiol. 50, 211–221 (2018).
Yang, W., Carrillo-Reid, L., Bando, Y., Peterka, D. S. & Yuste, R. Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. Elife 7, e32671 (2018).
Dal Maschio, M. et al. Simultaneous two-photon imaging and photo-stimulation with structured light illumination. Opt. Express 18, 18720–18731 (2010).
Nikolenko, V. et al. SLM Microscopy: scanless two-photon imaging and photostimulation with spatial light modulators. Front. Neural Circuits 2, 5 (2008).
Goodman, J. W. Introduction to Fourier Optics (W. H. Freeman, New York, 2005).
Curtis, J. E., Koss, B. A. & Grier, D. G. Dynamic holographic optical tweezers. Opt. Commun. 207, 169–175 (2002).
Zhao, Y. et al. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 (2011).
Dana, H. et al. Sensitive red protein calcium indicators for imaging neural activity. Elife 5, e12727 (2016).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Berlin, S. et al. Photoactivatable genetically encoded calcium indicators for targeted neuronal imaging. Nat. Methods 12, 852–858 (2015).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Habermacher, C. et al. Photo-switchable tweezers illuminate pore-opening motions of an ATP-gated P2X ion channel. Elife 5, e11050 (2016).
Lester, H. A. & Chang, H. W. Response of acetylcholine receptors to rapid photochemically produced increases in agonist concentration. Nature 266, 373–374 (1977).
Borowiak, M. et al. Photoswitchable inhibitors of microtubule dynamics optically control mitosis and cell death. Cell 162, 403–411 (2015).
Gaub, B. M. et al. Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Proc. Natl. Acad. Sci. USA 111, E5574–E5583 (2014).
Tochitsky, I., Kienzler, M. A., Isacoff, E. & Kramer, R. H. Restoring vision to the blind with chemical photoswitches. Chem. Rev. 118, 10748–10773 (2018).
Baker, C. K. & Flannery, J. G. Innovative optogenetic strategies for vision restoration. Front. Cell. Neurosci. 12, 316 (2018).
Guenthner, C. J., Miyamichi, K., Yang, H. H., Heller, H. C. & Luo, L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784 (2013).
Kawashima, T., Okuno, H. & Bito, H. A new era for functional labeling of neurons: activity-dependent promoters have come of age. Front. Neural Circuits 8, 37 (2014).
Ryan, T. J., Roy, D. S., Pignatelli, M., Arons, A. & Tonegawa, S. Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Tonegawa, S., Liu, X., Ramirez, S. & Redondo, R. Memory engram cells have come of age. Neuron 87, 918–931 (2015).
Zhou, Q. & Sheng, M. NMDA receptors in nervous system diseases. Neuropharmacology 74, 69–75 (2013).
Milnerwood, A. J. et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron 65, 178–190 (2010).
Okamoto, S. et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat. Med. 15, 1407–1413 (2009).
Tischer, D. & Weiner, O. D. Illuminating cell signalling with optogenetic tools. Nat. Rev. Mol. Cell Biol. 15, 551–558 (2014).
Govorunova, E. G., Sineshchekov, O. A., Li, H. & Spudich, J. L. Microbial rhodopsins: diversity, mechanisms, and optogenetic applications. Annu. Rev. Biochem. 86, 845–872 (2017).
Yawo, H., Asano, T., Sakai, S. & Ishizuka, T. Optogenetic manipulation of neural and non-neural functions. Dev. Growth Differ. 55, 474–490 (2013).
Wen, L. et al. Opto-current-clamp actuation of cortical neurons using a strategically designed channelrhodopsin. PLoS ONE 5, e12893 (2010).
Quejada, J. R. et al. Optimized light-inducible transcription in mammalian cells using Flavin Kelch-repeat F-box1/GIGANTEA and CRY2/CIB1. Nucleic Acids Res. 45, e172 (2017).
Broussard, G. J. et al. In vivo measurement of afferent activity with axon-specific calcium imaging. Nat. Neurosci. 21, 1272–1280 (2018).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Mahn, M. et al. High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins. Nat. Commun. 9, 4125 (2018).
Zhang, F. et al. The microbial opsin family of optogenetic tools. Cell 147, 1446–1457 (2011).
Thalhammer, G., Bowman, R. W., Love, G. D., Padgett, M. J. & Ritsch-Marte, M. Speeding up liquid crystal SLMs using overdrive with phase change reduction. Opt. Express 21, 1779–1797 (2013).
Vaziri, A. & Emiliani, V. Reshaping the optical dimension in optogenetics. Curr. Opin. Neurobiol. 22, 128–137 (2012).
Mathieson, T. et al. Systematic analysis of protein turnover in primary cells. Nat. Commun. 9, 689 (2018).
Cohen, L. D. et al. Metabolic turnover of synaptic proteins: kinetics, interdependencies and implications for synaptic maintenance. PLoS ONE 8, e63191 (2013).
Drobizhev, M., Makarov, N. S., Tillo, S. E., Hughes, T. E. & Rebane, A. Two-photon absorption properties of fluorescent proteins. Nat. Methods 8, 393–399 (2011).
Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010).
Lutz, C. et al. Holographic photolysis of caged neurotransmitters. Nat. Methods 5, 821–827 (2008).
Bègue, A. et al. Two-photon excitation in scattering media by spatiotemporally shaped beams and their application in optogenetic stimulation. Biomed. Opt. Express 4, 2869–2879 (2013).
Papagiakoumou, E., de Sars, V., Oron, D. & Emiliani, V. Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses. Opt. Express 16, 22039–22047 (2008).
Hernandez, O. et al. Three-dimensional spatiotemporal focusing of holographic patterns. Nat. Commun. 7, 11928 (2016).
Pégard, N. C. et al. Three-dimensional scanless holographic optogenetics with temporal focusing (3D-SHOT). Nat. Commun. 8, 1228 (2017).
Mardinly, A. R. et al. Precise multimodal optical control of neural ensemble activity. Nat. Neurosci. 21, 881–893 (2018).
Zhang, J. Z., Pegard, N., Zhong, J. S., Adesnik, H. & Waller, L. 3D computer-generated holography by non-convex optimization. Optica 4, 1306–1313 (2017).
Di Leonardo, R., Ianni, F. & Ruocco, G. Computer generation of optimal holograms for optical trap arrays. Opt. Express 15, 1913–1922 (2007).
Pozzi, P. et al. Fast calculation of computer generated holograms for 3D photostimulation through compressive-sensing Gerchberg–Saxton algorithm. Methods Protoc. 2, 2 (2019).
Carbone, A. L. & Plested, A. J. Coupled control of desensitization and gating by the ligand binding domain of glutamate receptors. Neuron 74, 845–857 (2012).
Weston, M. C., Gertler, C., Mayer, M. L. & Rosenmund, C. Interdomain interactions in AMPA and kainate receptors regulate affinity for glutamate. J. Neurosci. 26, 7650–7658 (2006).
Levitz, J., Popescu, A. T., Reiner, A. & Isacoff, E. Y. A toolkit for orthogonal and in vivo optical manipulation of ionotropic glutamate receptors. Front. Mol. Neurosci. 9, 2 (2016).
Mah, S. J., Cornell, E., Mitchell, N. A. & Fleck, M. W. Glutamate receptor trafficking: endoplasmic reticulum quality control involves ligand binding and receptor function. J. Neurosci. 25, 2215–2225 (2005).
Al-Ali, H., Blackmore, M., Bixby, J. L. & Lemmon, V. P. in Assay Guidance Manual (eds. Sittampalam, G. S. et al.) (Eli Lilly & Company and the National Center for Advancing Translational Sciences, Bethesda, MD, 2004).
Coussens, N. P. et al. Assay guidance manual: quantitative biology and pharmacology in preclinical drug discovery. Clin. Transl. Sci. 11, 461–470 (2018).
Timm, M., Saaby, L., Moesby, L. & Hansen, E. W. Considerations regarding use of solvents in in vitro cell based assays. Cytotechnology 65, 887–894 (2013).
Molleman, A. Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology (Wiley, Chichester, UK, 2003).
Miyazaki, J. et al. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene 79, 269–277 (1989).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991).
Kugler, S., Kilic, E. & Bahr, M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 10, 337–347 (2003).
Besnard, F. et al. Multiple interacting sites regulate astrocyte-specific transcription of the human gene for glial fibrillary acidic protein. J. Biol. Chem. 266, 18877–18883 (1991).
Dascal, N. & Kahanovitch, U. The roles of Gβγ and Gα in gating and regulation of GIRK channels. Int. Rev. Neurobiol. 123, 27–85 (2015).
Dascal, N. Signalling via the G protein-activated K+ channels. Cell. Signal. 9, 551–573 (1997).
Kahanovitch, U., Berlin, S. & Dascal, N. Collision coupling in the GABAB receptor-G protein-GIRK signaling cascade. FEBS Lett. 591, 2816–2825 (2017).
Vorobiov, D., Bera, A. K., Keren-Raifman, T., Barzilai, R. & Dascal, N. Coupling of the muscarinic m2 receptor to G protein-activated K(+) channels via Gα(z) and a receptor-Gα(z) fusion protein. Fusion between the receptor and Gα(z) eliminates catalytic (collision) coupling. J. Biol. Chem. 275, 4166–4170 (2000).
Reiter, A., Skerra, A., Trauner, D. & Schiefner, A. A photoswitchable neurotransmitter analogue bound to its receptor. Biochemistry 52, 8972–8974 (2013).
Rickgauer, J. P. & Tank, D. W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl. Acad. Sci. USA 106, 15025–15030 (2009).
Feldbauer, K. et al. Channelrhodopsin-2 is a leaky proton pump. Proc. Natl. Acad. Sci. USA 106, 12317–12322 (2009).
Lin, J. Y., Lin, M. Z., Steinbach, P. & Tsien, R. Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814 (2009).
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100, 13940–13945 (2003).
Zhang, F. et al. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat. Neurosci. 11, 631–633 (2008).
Schneider, F., Gradmann, D. & Hegemann, P. Ion selectivity and competition in channelrhodopsins. Biophys. J. 105, 91–100 (2013).
Prakash, R. et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods 9, 1171–1179 (2012).
Fenno, L., Yizhar, O. & Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412 (2011).
Wollmuth, L. P. et al. The lurcher mutation identifies delta 2 as an AMPA/kainate receptor-like channel that is potentiated by Ca2+. J. Neurosci. 20, 5973–5980 (2000).
Wollmuth, L. P. & Sakmann, B. Different mechanisms of Ca2+ transport in NMDA and Ca2+-permeable AMPA glutamate receptor channels. J. Gen. Physiol. 112, 623–636 (1998).
Acknowledgements
Support was provided by the Israel Science Foundation (S.B.; 1096/17 and 438/18). The research submitted was in partial fulfillment of the degree of master of science for I.C. and M.D.B.
Author information
Authors and Affiliations
Contributions
E.C.C. developed the 2P protocols; M.A.K. designed and synthesized all photoswitches. S.B. performed genetic modifications. S.B. and E.C.C. performed electrophysiology and Ca2+-imaging experiments; S.B., E.C.C. and M.A.K. designed the research project. I.C., M.D.B., L.M., E.C.C., M.A.K. and S.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information Nature Protocols thanks Pau Gorostiza Langa and other (anonymous) reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Levitz, J. et al. Nat. Neurosci. 16, 507–516: https://doi.org/10.1038/nn.3346 (2013)
Berlin, S. et al. Elife 5, e12040: https://doi.org/10.7554/eLife.12040 (2016)
Key data used in this protocol
Carroll, E. C. et al. Proc. Natl. Acad. Sci. USA 112, E776–E785: https://doi.org/10.1073/pnas.1416942112 (2015)
Kienzler, M. A. et al. J. Am. Chem. Soc. 135, 17683–17686: https://doi.org/10.1021/ja408104w (2013)
Supplementary information
Rights and permissions
About this article
Cite this article
Carmi, I., De Battista, M., Maddalena, L. et al. Holographic two-photon activation for synthetic optogenetics. Nat Protoc 14, 864–900 (2019). https://doi.org/10.1038/s41596-018-0118-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0118-2
This article is cited by
-
Polarized branched Actin modulates cortical mechanics to produce unequal-size daughters during asymmetric division
Nature Cell Biology (2023)
-
New tricks and emerging applications from contemporary azobenzene research
Photochemical & Photobiological Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.