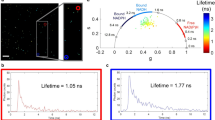
Abstract
Extracellular vesicles (EVs) are highly specialized nanoscale assemblies that deliver complex biological cargos to mediate intercellular communication. EVs are heterogeneous, and characterization of this heterogeneity is paramount to understanding EV biogenesis and activity, as well as to associating them with biological responses and pathologies. Traditional approaches to studying EV composition generally lack the resolution and/or sensitivity to characterize individual EVs, and therefore the assessment of EV heterogeneity has remained challenging. We have recently developed an atomic force microscope IR spectroscopy (AFM-IR) approach to probe the structural composition of single EVs with nanoscale resolution. Here, we provide a step-by-step procedure for our approach and show its power to reveal heterogeneity across individual EVs, within the same population of EVs and between different EV populations. Our approach is label free and able to detect lipids, proteins and nucleic acids within individual EVs. After isolation of EVs from cell culture medium, the protocol involves incubation of the EV sample on a suitable substrate, setup of the AFM-IR instrument and collection of nano-IR spectra and nano-IR images. Data acquisition and analyses can be completed within 24 h, and require only a basic knowledge of spectroscopy and chemistry. We anticipate that new understanding of EV composition and structure through AFM-IR will contribute to our biological understanding of EV biology and could find application in disease diagnosis and the development of EV therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information.
References
Barile, L. & Vassalli, G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 174, 63–78 (2017).
Tkach, M. & Théry, C. Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232 (2016).
Iraci, N., Leonardi, T., Gessler, F., Vega, B. & Pluchino, S. Focus on extracellular vesicles: physiological role and signalling properties of extracellular membrane vesicles. Int. J. Mol. Sci. 17, 171 (2016).
Uta, E. & Joanne, L. Analytical challenges of extracellular vesicle detection: a comparison of different techniques. Cytometry Part A 89, 123–134 (2016).
Jørgensen, M. et al. Extracellular vesicle (EV) array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J. Extracell. Vesicles 2, 20920 (2013).
Wang, X. et al. Unique molecular profile of exosomes derived from primary human proximal tubular epithelial cells under diseased conditions. J. Extracell. Vesicles 6, 1314073 (2017).
Jakobsen, K. R. et al. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J. Extracell. Vesicles 4, 26659 (2015).
Kowal, J. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 113, E968–E977 (2016).
De Toro, J., Herschlik, L., Waldner, C. & Mongini, C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front. Immunol. 6, 203 (2015).
Revenfeld, A. L. et al. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin. Ther. 36, 830–846 (2014).
Kim, S. Y., Khanal, D., Tharkar, P., Kalionis, B. & Chrzanowski, W. None of us is the same as all of us: resolving the heterogeneity of extracellular vesicles using single-vesicle, nanoscale characterization with resonance enhanced atomic force microscope infrared spectroscopy (AFM-IR). Nanoscale Horiz. 3, 430–438 (2018).
Minciacchi, V. R., Zijlstra, A., Rubin, M. A. & Di Vizio, D. Extracellular vesicles for liquid biopsy in prostate cancer: where are we and where are we headed? Prostate Cancer Prostatic Dis. 20, 251–258 (2017).
Armstrong, D. & Wildman, D. E. Extracellular vesicles and the promise of continuous liquid biopsies. J. Pathol. Transl. Med. 52, 1–8 (2018).
Stanko, P., Altanerova, U., Jakubechova, J., Repiska, V. & Altaner, C. Dental mesenchymal stem/stromal cells and their exosomes. Stem Cells Int. 2018, 8973613 (2018).
Zhu, Q., Heon, M., Zhao, Z. & He, M. Microfluidic engineering of exosomes: editing cellular messages for precision therapeutics. Lab Chip 18, 1690–1703 (2018).
Ye, Z. et al. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl. Mater. Interfaces 10, 12341–12350 (2018).
Yang, T. et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 32, 2003–2014 (2015).
Willms, E. et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 6, 22519 (2016).
Giebel, B. On the function and heterogeneity of extracellular vesicles. Ann. Transl. Med. 5, 150 (2017).
Hammiche, A. et al. Photothermal FT-IR spectroscopy: a step towards FT-IR microscopy at a resolution better than the diffraction limit. Appl. Spectrosc. 53, 810–815 (1999).
Anderson, M. S. Infrared spectroscopy with an atomic force microscope. Appl. Spectrosc. 54, 349–352 (2000).
Dazzi, A., Prazeres, R., Glotin, F. & Ortega, J. M. Local infrared microspectroscopy with subwavelength spatial resolution with an atomic force microscope tip used as a photothermal sensor. Opt. Lett. 30, 2388–2390 (2005).
Xiao, L. & Schultz, Z. D. Spectroscopic imaging at the nanoscale: technologies and recent applications. Anal. Chem. 90, 440–458 (2018).
Dazzi, A. & Prater, C. B. AFM-IR: technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem. Rev. 117, 5146–5173 (2017).
Centrone, A. Infrared imaging and spectroscopy beyond the diffraction limit. Annu. Rev. Anal. Chem. 8, 101–126 (2015).
Hinrichs, K. & Shaykhutdinov, T. Polarization-dependent atomic force microscopy-infrared spectroscopy (AFM-IR): infrared nanopolarimetric analysis of structure and anisotropy of thin films and surfaces. Appl. Spectrosc. 72, 817–832 (2018).
Khanal, D. et al. Biospectroscopy of nanodiamond-induced alterations in conformation of intra- and extracellular proteins: a nanoscale IR study. Anal. Chem. 88, 7530–7538 (2016).
Quaroni, L., Pogoda, K., Wiltowska-Zuber, J. & Kwiatek, W. M. Mid-infrared spectroscopy and microscopy of subcellular structures in eukaryotic cells with atomic force microscopy-infrared spectroscopy. RSC Adv. 8, 2786–2794 (2018).
Dazzi, A., Glotin, F. & Carminati, R. Theory of infrared nanospectroscopy by photothermal induced resonance. J. Appl. Phys. 107, 124519 (2010).
Qin, S. Q. et al. Establishment and characterization of fetal and maternal mesenchymal stem/stromal cell lines from the human term placenta. Placenta 39, 134–146 (2016).
Kusuma, G. D. et al. Mesenchymal stem/stromal cells derived from a reproductive tissue niche under oxidative stress have high aldehyde dehydrogenase activity. Stem Cell Rev. Rep. 12, 285–297 (2016).
Borges, F. T., Reis, L. A. & Schor, N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 46, 824–830 (2013).
Thakur, B. K. et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 24, 766–769 (2014).
Chen, I. H. et al. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc. Natl. Acad. Sci. USA 114, 3175–3180 (2017).
Baker, M. J. et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 9, 1771–1791 (2014).
McCarthy, S. A., Davies, G.-L. & Gun’ko, Y. K. Preparation of multifunctional nanoparticles and their assemblies. Nat. Protoc. 7, 1677–1693 (2012).
Martin, F. L. et al. Distinguishing cell types or populations based on the computational analysis of their infrared spectra. Nat. Protoc. 5, 1748–1760 (2010).
Dazzi, A. et al. AFM–IR: combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl. Spectrosc. 66, 1365–1384 (2012).
Butler, H. J. et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 11, 664–687 (2016).
Zhang, Y., Zhao, S., Zheng, J. & He, L. Surface-enhanced Raman spectroscopy (SERS) combined techniques for high-performance detection and characterization. Trends Anal. Chem. 90, 1–13 (2017).
Tatischeff, I., Larquet, E., Falcón-Pérez, J. M., Turpin, P.-Y. & Kruglik, S. G. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J. Extracell. Vesicles 1, 19179 (2012).
Park, J. et al. Exosome classification by pattern analysis of surface-enhanced Raman spectroscopy data for lung cancer diagnosis. Anal. Chem. 89, 6695–6701 (2017).
Grasso, L., Wyss, R. & Vogel, H. Use of fourier transform infrared spectroscopy analysis of extracellular vesicles isolated from body fluids for diagnosing, prognosing and monitoring pathophysiological states and method therfor. EPFL patent WO2016097996 (A1) (2016).
Ramer, G., Aksyuk, V. A. & Centrone, A. Quantitative chemical analysis at the nanoscale using the photothermal induced resonance technique. Anal. Chem. 89, 13524–13531 (2017).
Jaffar, J. et al. Greater cellular stiffness in fibroblasts from patients with idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 315, L59–L65 (2018).
Mihály, J. et al. Characterization of extracellular vesicles by IRspectroscopy: fast and simple classification based on amide and CH stretching vibrations. Biochim. Biophys. Acta Biomembr. 1859, 459–466 (2017).
Acknowledgements
We acknowledge the University of Sydney for the SOAR Fellowship for W.C. We acknowledge K. Kjoller, Photothermal Spectroscopy Corporation, for consultation and technical support.
Author information
Authors and Affiliations
Contributions
W.C. conceived, designed and oversaw the project; S.Y.K. co-designed experiments and performed the isolation of EVs; D.K. performed the AFM-IR; and B.K. isolated and developed the stem cell lines. S.Y.K., D.K., B.K. and W.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Kim, S. Y., Khanal, D., Tharkar, P., Kalionis, B. & Chrzanowski, W. Nanoscale Horiz. 3, 430–438 (2018): https://doi.org/10.1039/C8NH00048D
Khanal, D. et al. Anal. Chem. 88, 7530–7538 (2016): https://doi.org/10.1021/acs.analchem.6b00665
Khanal, D. et al. Part. Part. Syst. Charact. 35, 1700409 (2018): https://doi.org/10.1002/ppsc.201700409
Integrated supplementary information
Supplementary Figure 1 Troubleshooting: examples of possible errors that can occur if critical steps are not properly implemented in this protocol.
(a) Image of an EV sample on ZnSe prism with crystals formed from the drying of the sample with residual phosphate buffer saline (PBS); (b) Atomic force microscopy (AFM) height image of an EV sample without proper immobilization, causing dragging during imaging; (c) AFM height image and corresponding AFM-IR spectra of EV sample, where laser was misaligned and had to be re-optimized before correct acquisition of the spectra.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1
Rights and permissions
About this article
Cite this article
Kim, S.Y., Khanal, D., Kalionis, B. et al. High-fidelity probing of the structure and heterogeneity of extracellular vesicles by resonance-enhanced atomic force microscopy infrared spectroscopy. Nat Protoc 14, 576–593 (2019). https://doi.org/10.1038/s41596-018-0109-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0109-3
This article is cited by
-
Extracellular vesicles derived from mesenchymal stem cells — a novel therapeutic tool in infectious diseases
Inflammation and Regeneration (2023)
-
Infrared nanospectroscopic imaging of DNA molecules on mica surface
Scientific Reports (2022)
-
Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles
Nature Protocols (2021)
-
Deconvolution of dissipative pathways for the interpretation of tapping-mode atomic force microscopy from phase-contrast
Communications Physics (2021)
-
Nanomechanical and Chemical Mapping of the Structure and Interfacial Properties in Immiscible Ternary Polymer Systems
Chinese Journal of Polymer Science (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.