Abstract
Despite decades of research, pharmacological therapies for spinal cord motor pathologies are limited. Alternatives using macromolecular, viral, or cell-based therapies show early promise. However, introducing these substances into the spinal cord, past the blood–brain barrier, without causing injury is challenging. We describe a technique for intraspinal injection targeting the lumbar ventral horn in rodents. This technique preserves motor performance and has a proven track record of translation into phase 1 and 2 clinical trials in amyotrophic lateral sclerosis (ALS) patients. The procedure, in brief, involves exposure of the thoracolumbar spine and dissection of paraspinous muscles over the target vertebrae. Following laminectomy, the spine is affixed to a stereotactic frame, permitting precise and reproducible injection throughout the lumbar spine. We have used this protocol to inject various stem cell types, primarily human spinal stem cells (HSSCs); however, the injection is adaptable to any candidate therapeutic cell, virus, or macromolecule product. In addition to a detailed procedure, we provide stereotactic coordinates that assist in targeting of the lumbar spine and instructional videos. The protocol takes ~2 h per animal.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data presented with the protocol are available from the corresponding author on reasonable request.
References
Ashizawa, T., Oz, G. & Paulson, H. L. Spinocerebellar ataxias: prospects and challenges for therapy development. Nat. Rev. Neurol. 14, 590–605 (2018).
Groen, E. J. N., Talbot, K. & Gillingwater, T. H. Advances in therapy for spinal muscular atrophy: promises and challenges. Nat. Rev. Neurol. 14, 214–224 (2018).
Hardiman, O. et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 3, 17071 (2017).
Teoh, H. L. et al. Inherited paediatric motor neuron disorders: beyond spinal muscular atrophy. Neural Plast. 2017, 6509493 (2017).
Ahuja, C. S. et al. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 3, 17018 (2017).
Langhorne, P., Coupar, F. & Pollock, A. Motor recovery after stroke: a systematic review. Lancet Neurol. 8, 741–754 (2009).
Gubert, F. et al. Intraspinal bone-marrow cell therapy at pre- and symptomatic phases in a mouse model of amyotrophic lateral sclerosis. Stem Cell Res. Ther. 7, 41 (2016).
Inquimbert, P., Moll, M., Kohno, T. & Scholz, J. Stereotaxic injection of a viral vector for conditional gene manipulation in the mouse spinal cord. J. Vis. Exp. 2013, e50313 (2013).
Knippenberg, S. et al. Intraspinal administration of human spinal cord-derived neural progenitor cells in the G93A-SOD1 mouse model of ALS delays symptom progression, prolongs survival and increases expression of endogenous neurotrophic factors. J. Tissue Eng. Regen. Med. 11, 751–764 (2017).
Kohro, Y. et al. A new minimally-invasive method for microinjection into the mouse spinal dorsal horn. Sci. Rep. 5, 14306 (2015).
Lepore, A. C. Intraspinal cell transplantation for targeting cervical ventral horn in amyotrophic lateral sclerosis and traumatic spinal cord injury. J. Vis. Exp. 2011, 3069 (2011).
Hefferan, M. P. et al. Human neural stem cell replacement therapy for amyotrophic lateral sclerosis by spinal transplantation. PLoS ONE 7, e42614 (2012).
Xu, L., Ryugo, D. K., Pongstaporn, T., Johe, K. & Koliatsos, V. E. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J. Comp. Neurol. 514, 297–309 (2009).
Xu, L., Shen, P., Hazel, T., Johe, K. & Koliatsos, V. E. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci. Lett. 494, 222–226 (2011).
Xu, L. et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation 82, 865–875 (2006).
Yan, J. et al. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells 24, 1976–1985 (2006).
Yan, J. et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 4, e39 (2007).
Goutman, S. A. et al. Long-term phase 1/2 intraspinal stem cell transplantation outcomes in ALS. Ann. Clin. Transl. Neurol. 5, 730–740 (2018).
Feldman, E. L. et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: Phase 1 trial outcomes. Ann. Neurol. 75, 363–373 (2014).
Glass, J. D. et al. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells 30, 1144–1151 (2012).
Glass, J. D. et al. Transplantation of spinal cord-derived neural stem cells for ALS: analysis of phase 1 and 2 trials. Neurology 87, 392–400 (2016).
Riley, J. et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery 71, 405–416 (2012). discussion 416.
Riley, J. et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery 74, 77–87 (2014).
Hardcastle, N., Boulis, N. M. & Federici, T. AAV gene delivery to the spinal cord: serotypes, methods, candidate diseases, and clinical trials. Expert Opin. Biol. Ther. 18, 293–307 (2018).
Schoch, K. M. & Miller, T. M. Antisense oligonucleotides: translation from mouse models to human neurodegenerative diseases. Neuron 94, 1056–1070 (2017).
Cizkova, D. et al. Functional recovery in rats with ischemic paraplegia after spinal grafting of human spinal stem cells. Neuroscience 147, 546–560 (2007).
Gordon, P. H. Amyotrophic lateral sclerosis: an update for 2013 clinical features, pathophysiology, management and therapeutic trials. Aging Dis. 4, 295–310 (2013).
Bensimon, G., Lacomblez, L. & Meininger, V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 330, 585–591 (1994).
Writing Group & Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16, 505–512 (2017).
Chen, K. S., Sakowski, S. A. & Feldman, E. L. Intraspinal stem cell transplantation for amyotrophic lateral sclerosis. Ann. Neurol. 79, 342–353 (2016).
Lunn, J. S., Sakowski, S. A. & Feldman, E. L. Stem cell therapies for amyotrophic lateral sclerosis: Recent advances and prospects for the future. Stem Cells 32, 1099–1109 (2014).
Sakowski, S. A., Lunn, J. S. & Feldman, E. L. Stem cell therapy for motor neuron disease. in Motor Neuron Disease in Adults, Contemporary Neurology Series, Vol. 88 (eds Bruijn, L. & Bromberg, M. B.) 312–317 (Oxford University Press, New York, 2015).
Ilieva, H., Polymenidou, M. & Cleveland, D. W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 187, 761–772 (2009).
Paez-Colasante, X., Figueroa-Romero, C., Sakowski, S. A., Goutman, S. A. & Feldman, E. L. Amyotrophic lateral sclerosis: mechanisms and therapeutics in the epigenomic era. Nat. Rev. Neurol. 11, 266–279 (2015).
Boulis, N. M. et al. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat. Rev. Neurol. 8, 172–176 (2011).
Goutman, S. A., Chen, K. S. & Feldman, E. L. Recent advances and the future of stem cell therapies in amyotrophic lateral sclerosis. Neurotherapeutics 12, 428–448 (2015).
Thomsen, G. M., Gowing, G., Svendsen, S. & Svendsen, C. N. The past, present and future of stem cell clinical trials for ALS. Exp. Neurol. 262, 127–137 (2014).
Johe, K. K., Hazel, T. G., Muller, T., Dugich-Djordjevic, M. M. & McKay, R. D. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 10, 3129–3140 (1996).
Raore, B. et al. Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine 36, E164–E171 (2011).
Riley, J. et al. Targeted spinal cord therapeutics delivery: stabilized platform and MER guidance validation. Stereotact. Funct. Neurosurg. 86, 67–74 (2007).
Riley, J. et al. Cervical spinal cord therapeutics delivery: preclinical safety validation of a stabilized microinjection platform. Neurosurgery 65, 754–761 (2009). discussion 761-762.
Riley, J. P., Raore, B., Taub, J. S., Federici, T. & Boulis, N. M. Platform and cannula design improvements for spinal cord therapeutics delivery. Neurosurgery 69, 147–155 (2011).
Usvald, D. et al. Analysis of dosing regimen and reproducibility of intraspinal grafting of human spinal stem cells in immunosuppressed minipigs. Cell Transplant. 19, 1103–1122 (2010).
Hefferan, M. P. et al. Optimization of immunosuppressive therapy for spinal grafting of human spinal stem cells in a rat model of ALS. Cell Transplant. 20, 1153–1161 (2011).
McGoldrick, P., Joyce, P. I., Fisher, E. M. & Greensmith, L. Rodent models of amyotrophic lateral sclerosis. Biochim. Biophys. Acta 1832, 1421–1436 (2013).
Philips, T. & Rothstein, J. D. Rodent models of amyotrophic lateral sclerosis. Curr. Protoc. Pharmacol. 69, 5.67.1–5.67.21 (2015).
Xu, L., Shen, P., Hazel, T., Johe, K. & Koliatsos, V. E. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci. Lett. 494, 222–226 (2011).
Franz, C. K. et al. Intraspinal cord delivery of IGF-I mediated by adeno-associated virus 2 is neuroprotective in a rat model of familial ALS. Neurobiol. Dis. 33, 473–481 (2009).
Lepore, A. C. et al. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res. 1185, 256–265 (2007).
Donnelly, E. M. et al. Lentiviral vector delivery of short hairpin RNA to NG2 and neurotrophin-3 promotes locomotor recovery in injured rat spinal cord. Cytotherapy 14, 1235–1244 (2012).
Macks, C., Gwak, S. J., Lynn, M. & Lee, J. S. Rolipram-loaded polymeric micelle nanoparticle reduces secondary injury after rat compression spinal cord injury. J. Neurotrauma 35, 582–592 (2018).
Povysheva, T. et al. Post-spinal cord injury astrocyte-mediated functional recovery in rats after intraspinal injection of the recombinant adenoviral vectors Ad5-VEGF and Ad5-ANG. J. Neurosurg. Spine 27, 105–115 (2017).
Santamaria, A. J., Solano, J. P., Benavides, F. D. & Guest, J. D. Intraspinal delivery of Schwann cells for spinal cord injury. Methods Mol. Biol. 1739, 467–484 (2018).
Brown, R. H. Jr. & Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 377, 162–172 (2017).
Parente, V. & Corti, S. Advances in spinal muscular atrophy therapeutics. Ther. Adv. Neurol. Disord. 11, 1756285618754501 (2018).
Scoles, D. R. et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature 544, 362–366 (2017).
Thuret, S., Moon, L. D. & Gage, F. H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 7, 628–643 (2006).
Garbuzova-Davis, S. et al. Positive effect of transplantation of hNT neurons (NTera 2/D1 cell-line) in a model of familial amyotrophic lateral sclerosis. Exp. Neurol. 174, 169–180 (2002).
Bell, J. A., Sharpe, L. G. & Berry, J. N. Depressant and excitant effects of intraspinal microinjections of morphine and methionine-enkephalin in the cat. Brain Res. 196, 455–465 (1980).
Garbuzova-Davis, S. et al. Intraspinal implantation of hNT neurons into SOD1 mice with apparent motor deficit. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2, 175–180 (2001).
Qiu, K., Falk, D. J., Reier, P. J., Byrne, B. J. & Fuller, D. D. Spinal delivery of AAV vector restores enzyme activity and increases ventilation in Pompe mice. Mol. Ther. 20, 21–27 (2012).
Dutta, S. & Sengupta, P. Men and mice: relating their ages. Life Sci. 152, 244–248 (2016).
Sengupta, P. The laboratory rat: relating its age with human’s. Int. J. Prev. Med. 4, 624–630 (2013).
Lunn, J. S. et al. Intraspinal transplantation of neurogenin-expressing stem cells generates spinal cord neural progenitors. Neurobiol. Dis. 46, 59–68 (2012).
Acknowledgements
We thank C. Backus, C. Pacut, and M. Sweeney for technical support. This work was supported by the Sinai Medical Foundation, the A. Alfred Taubman Medical Research Institute, the Program for Neurology Research & Discovery, University of Michigan Clinician Scientist Training Program grants NINDS R25NS089450 (K.S.C.) and NIH T32NS07222 (O.N.K.), and the Robert E. Nederlander Sr. Program for Alzheimer’s Research (L.M.M.).
Author information
Authors and Affiliations
Contributions
E.L.F., K.S.C., L.M.M., O.N.K, and K.J. conceived of and designed the experiments. K.S.C. and O.N.K. performed the surgery with J.M.H., E.S.B., and J.S.C. F.E.M. J.S.C., and M.A.T. performed behavioral testing. L.M.M., E.S.B., and J.M.H. performed tissue processing, histological analyses, and microscopy. K.S.C. and L.M.M. analyzed and interpreted the data, and E.L.F. supervised the study. K.S.C., L.M.M., and S.A.S wrote the manuscript, and all authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
K.J. is chief scientific officer for Neuralstem, which provided the stem cell product (NSI566-RSC) via a material transfer agreement. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Hefferan, M. P. et al. PLoS ONE 7, e42614 (2012): https://doi.org/10.1371/journal.pone.0042614
Yan, J. et al. Stem Cells 24, 1976–1985 (2006): https://doi.org/10.1634/stemcells.2005-0518
Lunn, J. S. et al. Neurobiol. Dis. 46, 59–68 (2012): https://doi.org/10.1016/j.nbd.2011.12.044
Integrated supplementary information
Supplementary Figure 1 Effect of intraspinal injection on animal survival and average number of surviving motor neurons.
Kaplan-Meier survival and quantification of postmortem motor neuron (MN) survival in wild type mice (a,b) and rats (c,d) undergoing vehicle intraspinal injection (WT Veh) versus animals undergoing no surgical procedure (WT). Motor neuron survival was assessed as described in the Supplementary Methods. Non-significant hazard ratio of mortality for animals undergoing intraspinal injection; minimal mortality (10% mortality for both mice (a) and rats (c) in the WT Veh animals could also be attributable to immunosuppressive regimen, as these animals served as controls in other stem cell experiments requiring immunosuppression. Likewise, quantified average number of surviving MN (mean ± SD) are not impacted by the injection protocol at approximately 180 days post-surgery, in mice (b; p=0.2762; WT vs. WT Veh; t-test) or in rats (d; p=0.1445; WT vs. WT Veh; t-test). MN = motor neuron; WT = wild-type; WT Veh = WT plus vehicle injection.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Methods
Supplementary Video 1
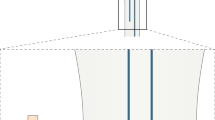
Technique for intraspinal injection of stem cells in SOD1G93A mouse lumbar spine. An incision is made over the lumbar area, and paraspinous muscles are sharply dissected free from spinous processes and spinal laminae. Cutting the ligamentous attachment to the facet joint facilitates exposure. By palpating the final rib, a laminectomy is performed at the T13 and L1 level using microscissors. The central spinal vein is used as the reference for midline. The needle tip is introduced 0.3 mm lateral to the 29 central vein and 0.8 mm deep to the dorsal surface of the spinal cord to reach the ventral gray matter. Five bilateral injections (10 total injections) are made with a 1.0-mm distance between each set of injections. All animal experiments were approved by the University of Michigan Institutional Animal Care and Use Committee and were performed in accordance with University of Michigan guidelines (accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International) and state and federal regulations.
Supplementary Video 2
Technique for intraspinal injection of stem cells in SOD1G93A rat lumbar spine. An incision is made over the lumbar area, and paraspinous muscles are sharply dissected free from spinous processes and spinal laminae. Cutting the ligamentous attachment to the facet joint facilitates exposure. By palpating the final rib, a laminectomy is performed at the T13 level using a diamond-tip drill. The central spinal vein is used as the reference for midline. The needle tip is introduced 0.5 mm lateral to the central vein and 1.5 mm deep to the dorsal surface of the spinal cord to reach the ventral gray matter. Ten bilateral injections (20 total injections) are made with 0.5-mm distance between each set of injections. Fasciae of the subcutaneous muscle and skin are closed with absorbable sutures. All animal experiments were approved by the University of Michigan Institutional Animal Care and Use Committee and were performed in accordance with University of Michigan guidelines (accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International) and state and federal regulations.
Rights and permissions
About this article
Cite this article
Chen, K.S., McGinley, L.M., Kashlan, O.N. et al. Targeted intraspinal injections to assess therapies in rodent models of neurological disorders. Nat Protoc 14, 331–349 (2019). https://doi.org/10.1038/s41596-018-0095-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0095-5
This article is cited by
-
IL-6 from cerebrospinal fluid causes widespread pain via STAT3-mediated astrocytosis in chronic constriction injury of the infraorbital nerve
Journal of Neuroinflammation (2024)
-
SU16f inhibits fibrotic scar formation and facilitates axon regeneration and locomotor function recovery after spinal cord injury by blocking the PDGFRβ pathway
Journal of Neuroinflammation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.