Abstract
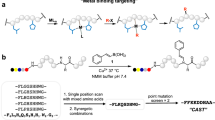
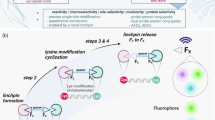
There is considerable interest in the development of chemical methods for the precise, site-selective modification of antibodies for therapeutic applications. In this protocol, we describe a strategy for the irreversible and selective modification of cysteine residues on antibodies, using functionalized carbonylacrylic reagents. This protocol is based on a thiol–Michael-type addition of native or engineered cysteine residues to carbonylacrylic reagents equipped with functional compounds such as cytotoxic drugs. This approach is a robust alternative to the conventional maleimide technique; the reaction is irreversible and uses synthetically accessible reagents. Complete conversion to the conjugates, with improved quality and homogeneity, is often achieved using a minimal excess (typically between 5 and 10 equiv.) of the carbonylacrylic reagent. Potential applications of this method cover a broad scope of cysteine-tagged antibodies in various formats (full-length IgGs, nanobodies) for the site-selective incorporation of cytotoxic drugs without loss of antigen-binding affinity. Both the synthesis of the carbonylacrylic reagent armed with a synthetic molecule of interest and the subsequent preparation of the chemically defined, homogeneous antibody conjugate can be achieved within 48 h and can be easily performed by nonspecialists. Importantly, the conjugates formed are stable in human plasma. The use of liquid chromatography–mass spectrometry (LC–MS) analysis is recommended for monitoring the progression of the bioconjugation reactions on protein and antibody substrates with accurate resolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Chudasama, V., Maruani, A. & Caddick, S. Recent advances in the construction of antibody–drug conjugates. Nat. Chem. 8, 114–119 (2016).
Xue, L., Karpenko, I. A., Hiblot, J. & Johnsson, K. Imaging and manipulating proteins in live cells through covalent labeling. Nat. Chem. Biol. 11, 917–923 (2015).
Rodrigues, T. & Bernardes, G. J. L. The missing link. Nat. Chem. 8, 1088–1090 (2016).
Krall, N., da Cruz, F. P., Boutureira, O. & Bernardes, G. J. L. Site-selective protein-modification chemistry for basic biology and drug development. Nat. Chem. 8, 103–113 (2015).
MacDonald, J. I., Munch, H. K., Moore, T. & Francis, M. B. One-step site-specific modification of native proteins with 2-pyridinecarboxyaldehydes. Nat. Chem. Biol. 11, 326–331 (2015).
Vinogradova, E. V., Zhang, C., Spokoyny, A. M., Pentelute, B. L. & Buchwald, S. L. Organometallic palladium reagents for cysteine bioconjugation. Nature 526, 687–691 (2015).
Boutureira, O. & Bernardes, G. J. L. Advances in chemical protein modification. Chem. Rev. 115, 2174–2195 (2015).
Matos, M. J. et al. Chemo- and regioselective lysine modification on native proteins. J. Am. Chem. Soc. 140, 4004–4017 (2018).
Beck, A., Goetsch, L., Dumontet, C. & Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 (2017).
Sievers, E. L. & Senter, P. D. Antibody-drug conjugates in cancer therapy. Ann. Rev. Med. 64, 15–29 (2013).
Bernardim, B. et al. Stoichiometric and irreversible cysteine-selective protein modification using carbonylacrylic reagents. Nat. Commun. 7, 13128 (2016).
Junutula, J. R. et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 26, 925–932 (2008).
Stenton, B. J., Oliveira, B. L., Matos, M. J., Sinatra, L. & Bernardes, G. J. L. A thioether-directed palladium-cleavable linker for targeted bioorthogonal drug decaging. Chem. Sci. 9, 4185–4189 (2018).
Rosen, C. B. & Francis, M. B. Targeting the N terminus for site-selective protein modification. Nat. Chem. Biol. 13, 697–705 (2017).
Chalker, J. M., Bernardes, G. J. L., Lin, Y. A. & Davis, B. G. Chemical modification of proteins at cysteine: opportunities in chemistry and biology. Chem. Asian J. 4, 630–640 (2009).
Pimenta Góis, P. M., Ravasco, J., Faustino, H. & Trindade, A. Bioconjugation with maleimides: a useful tool for chemical biology. Chem. Eur. J. doi:10.1002/chem.201803174 (2018).
Kalia, D., Malekar Pushpa, V. & Parthasarathy, M. Exocyclic olefinic maleimides: synthesis and application for stable and thiol‐selective bioconjugation. Angew. Chem. Int. Ed. Engl. 55, 1432–1435 (2015).
Lyon, R. P. et al. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nat. Biotechnol. 32, 1059–1062 (2014).
Vaneycken, I. et al. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 25, 2433–2446 (2011).
Lyon, R. P. et al. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat. Biotechnol. 33, 733–735 (2015).
Freedy, A. M. et al. Chemoselective installation of amine bonds on proteins through aza-Michael ligation. J. Am. Chem. Soc. 139, 18365–18375 (2017).
Levengood, M. R. et al. Orthogonal cysteine protection enables homogeneous multi-drug antibody–drug conjugates. Angew. Chem. Int. Ed. Engl. 56, 733–737 (2017).
Senter, P. D. & Sievers, E. L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 30, 631–637 (2012).
Carl, P. L., Chakravarty, P. K. & Katzenellenbogen, J. A. A novel connector linkage applicable in prodrug design. J. Med. Chem. 24, 479–480 (1981).
Cui, J. J. et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal–epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J. Med. Chem. 54, 6342–6363 (2011).
Brack, S. S., Silacci, M., Birchler, M. & Neri, D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-c. Clin. Cancer Res. 12, 3200–3208 (2006).
Heuveling, D. A. et al. Phase 0 microdosing PET study using the human mini antibody F16SIP in head and neck cancer patients. J. Nucl. Med. 54, 397–401 (2013).
Gébleux, R., Stringhini, M., Casanova, R., Soltermann, A. & Neri, D. Non‐internalizing antibody–drug conjugates display potent anti‐cancer activity upon proteolytic release of monomethyl auristatin E in the subendothelial extracellular matrix. Int. J. Cancer 140, 1670–1679 (2016).
Acknowledgements
We thank FAPESP (BEPE 2015/07509-1 and 2017/13168-8 to B.B., and 2013/25504-1 to A.C.B.B.), Xunta de Galicia (M.J.M.), FCT Portugal (FCT Investigator to G.J.L.B., IF/00624/2015), the EU (Marie Sklodowska-Curie ITN Protein Conjugates, GA 675007, including a PhD Studentship to X.F.), the Ministerio de Economía y Competitividad (projects CTQ2015-67727-R and UNLR13-4E-1931 to F.C. and CTQ2015-70524-R and RYC-2013-14706 to G.J.O.) and the Universidad de La Rioja (FPI Studentship to I.C.). We also thank S. Massa and N. Devoogdt (Vrije Universiteit Brussel (VUM), Brussels) for the generous gift of the Her2-targeting nanobody 2Rb17c, Genentech for providing Thiomab LC-V205C and trastuzumab antibodies, and D. Neri’s laboratory (Swiss Federal Institute of Technology (ETH Zürich), Zurich) for the generous gift of the F16 antibody. G.J.L.B. is a Royal Society University Research Fellow (UF110046 and URF/R/180019) and the recipient of a European Research Council Starting Grant (TagIt, GA 676832).
Author information
Authors and Affiliations
Contributions
G.J.L.B. designed and supervised the research. B.B. and M.J.M. performed reactions on antibodies and MS analysis. F.C., X.F. and I.C. designed and performed the synthesis of caa-ValCit-PAB-MMAE and caa-crizotinib. A.G. and P.A. studied antibody binding and performed flow cytometry analysis. A.C.B.B. and G.J.-O. contributed to the design of the carbonylacrylic reagent. B.B. and G.J.L.B. wrote the paper with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Stenton, B. J., Oliveira, B. L., Matos, M. J., Sinatra, L. & Bernardes, G. J. L. Chem. Sci. 9, 4185–4189 (2018): http://pubs.rsc.org/en/Content/ArticleLanding/2018/SC/C8SC00256H#!divAbstract
Bernardim, B. et al. Nat. Commun. 7, 13128 (2016): https://www.nature.com/articles/ncomms13128
Supplementary information
Supplementary Information
Supplementary Figures 1–25 and Supplementary Methods
Rights and permissions
About this article
Cite this article
Bernardim, B., Matos, M.J., Ferhati, X. et al. Efficient and irreversible antibody–cysteine bioconjugation using carbonylacrylic reagents. Nat Protoc 14, 86–99 (2019). https://doi.org/10.1038/s41596-018-0083-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0083-9
This article is cited by
-
Selenium chemistry for spatio-selective peptide and protein functionalization
Nature Reviews Chemistry (2024)
-
Controlled masking and targeted release of redox-cycling ortho-quinones via a C–C bond-cleaving 1,6-elimination
Nature Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.