Abstract
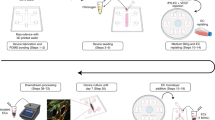
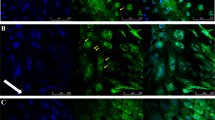
In vitro models of the blood–brain barrier (BBB) are critical tools for the study of BBB transport and the development of drugs that can reach the CNS. Brain endothelial cells grown in culture are often used to model the BBB; however, it is challenging to maintain reproducible BBB properties and function. ‘BBB organoids’ are obtained following coculture of endothelial cells, pericytes and astrocytes under low-adhesion conditions. These organoids reproduce many features of the BBB, including the expression of tight junctions, molecular transporters and drug efflux pumps, and hence can be used to model drug transport across the BBB. This protocol provides a comprehensive description of the techniques required to culture and maintain BBB organoids. We also describe two separate detection approaches that can be used to analyze drug penetration into the organoids: confocal fluorescence microscopy and mass spectrometry imaging. Using our protocol, BBB organoids can be established within 2–3 d. An additional day is required to analyze drug permeability. The BBB organoid platform represents an accurate, versatile and cost-effective in vitro tool. It can easily be scaled to a high-throughput format, offering a tool for BBB modeling that could accelerate therapeutic discovery for the treatment of various neuropathologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
DiLuca, M. & Olesen, J. The cost of brain diseases: a burden or a challenge? Neuron 82, 1205–1208 (2014).
Gooch, C. L., Pracht, E. & Borenstein, A. R. The burden of neurological disease in the United States: a summary report and call to action. Ann. Neurol. 81, 479–484 (2017).
Abbott, N. J., Rönnbäck, L. & Hansson, E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006).
Banks, W. A. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 15, 275–292 (2016).
Cho, C.-F. The blood–brain barrier: brain cancer therapy hits a wall. Oncol. Times 40, 1 (2018).
Cho, C.-F. et al. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 8, 15623 (2017).
Urich, E. et al. Multicellular self-assembled spheroidal model of the blood brain barrier. Sci. Rep. 3, 1500 (2013).
Wolburg, H. & Lippoldt, A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul. Pharmacol. 38, 323–337 (2002).
Begley, D. J. & Brightman, M. W. Structural and functional aspects of the blood–brain barrier. Prog. Drug Res. 61, 39–78 (2003).
Zlokovic, B. V. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008).
Hervé, F., Ghinea, N. & Scherrmann, J.-M. CNS delivery via adsorptive transcytosis. AAPS J. 10, 455–472 (2008).
Schinkel, A. H. P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv. Drug Deliv. Rev. 36, 179–194 (1999).
Rodriguez, A., Tatter, S. B. & Debinski, W. Neurosurgical techniques for disruption of the blood–brain barrier for glioblastoma treatment. Pharmaceutics 7, 175–187 (2015).
Aryal, M., Arvanitis, C. D., Alexander, P. M. & McDannold, N. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 72, 94–109 (2014).
Demeule, M. et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J. Neurochem. 106, 1534–1544 (2008).
Stalmans, S. et al. Cell-penetrating peptides selectively cross the blood–brain barrier in vivo. PLoS ONE 10, e0139652 (2015).
Zhang, Y.-Y., Liu, H., Summerfield, S. G., Luscombe, C. N. & Sahi, J. Integrating in silico and in vitro approaches to predict drug accessibility to the central nervous system. Mol. Pharm. 13, 1540–1550 (2016).
Bowman, P. D., Ennis, S. R., Rarey, K. E., Betz, A. L. & Goldstein, G. W. Brain microvessel endothelial cells in tissue culture: a model for study of blood-brain barrier permeability. Ann. Neurol. 14, 396–402 (1983).
Naik, P. & Cucullo, L. In vitro blood–brain barrier models: current and perspective technologies. J. Pharm. Sci. 101, 1337–1354 (2012).
Hatherell, K., Couraud, P.-O., Romero, I. A., Weksler, B. & Pilkington, G. J. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 199, 223–229 (2011).
Cecchelli, R. et al. A stable and reproducible human blood–brain barrier model derived from hematopoietic stem cells. PLoS ONE 9, e99733 (2014).
Ribecco-Lutkiewicz, M. et al. A novel human induced pluripotent stem cell blood-brain barrier model: applicability to study antibody-triggered receptor-mediated transcytosis. Sci. Rep. 8, 1873 (2018).
Holman, D. W., Klein, R. S. & Ransohoff, R. M. The blood–brain barrier, chemokines and multiple sclerosis. Biochim. Biophys. Acta 1812, 220–230 (2011).
Hudecz, D., Rocks, L., Fitzpatrick, L. W., Herda, L.-M. & Dawson, K. A. Reproducibility in biological models of the blood-brain barrier. Eur. J. Nanomed. 6, 185 (2014).
Griep, L. M. et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed. Microdevices 15, 145–150 (2013).
Booth, R. & Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 12, 1784–1792 (2012).
Cecchelli, R. et al. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 6, 650–661 (2007).
Fadzen, C. M. et al. Perfluoroarene-based peptide macrocycles to enhance penetration across the blood–brain barrier. J. Am. Chem. Soc. 139, 15628–15631 (2017).
Demeule, M. et al. Identification and design of peptides as a new drug delivery system for the brain. J. Pharmacol. Exp. Ther. 324, 1064–1072 (2008).
Maira, S.-M. et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol. Cancer Ther. 11, 317–328 (2012).
Liu, X. et al. Molecular imaging of drug transit through the blood-brain barrier with MALDI mass spectrometry imaging. Sci. Rep. 3, 2859 (2013).
Mittapalli, R. K., Vaidhyanathan, S., Dudek, A. Z. & Elmquist, W. F. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J. Pharmacol. Exp. Ther. 344, 655–664 (2013).
Zou, L.-L., Ma, J.-L., Wang, T., Yang, T.-B. & Liu, C.-B. Cell-penetrating peptide-mediated therapeutic molecule delivery into the central nervous system. Curr. Neuropharmacol. 11, 197–208 (2013).
Mijalis, A. J. et al. A fully automated flow-based approach for accelerated peptide synthesis. Nat. Chem. Biol. 13, 464–466 (2017).
Acknowledgements
C.-F.C. was supported by the Canadian Institute of Health Research Post-Doctoral Fellowship and is currently supported by a Brigham and Women’s Hospital Women’s Brain Initiative/Connors Center Pilot Grant as well as the Sperling Family Fellowship. N.Y.R.A. is supported by National Institutes of Health/National Cancer Institute (NIH/NCI) grants R01CA201469 and U54CA210180. B.L.P. is supported by the Sontag Distinguished Scientist Award. S.E.L. was supported by a B*CURED Research Grant and a Brigham and Women’s Hospital Women’s Innovation Award. We thank H. Bridger and H. Schulze from the Brigham and Women’s Research Institute for providing assistance with photography.
Author information
Authors and Affiliations
Contributions
C.-F.C., N.Y.R.A. and S.E.L. designed the research. C.-F.C. performed the experiments. S.B. and C.-F.C. analyzed the data. S.B., S.E.L., M.S.R. and C.-F.C. wrote the manuscript. C.M.F., J.M.W. and C.-F.C. revised the manuscript. S.B., S.E.L., Y.Q., C.M.F., J.M.W., M.S.R., B.L.P., N.Y.R.A. and C.-F.C. read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Cho, C. F. et al. Nat. Commun. 8, 15623 (2017): https://doi.org/10.1038/ncomms15623
Fadzen, C.M. J. Am. Chem. Soc. 139, 15628–15631 (2017): https://doi.org/10.1021/jacs.7b09790
Supplementary information
Rights and permissions
About this article
Cite this article
Bergmann, S., Lawler, S.E., Qu, Y. et al. Blood–brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc 13, 2827–2843 (2018). https://doi.org/10.1038/s41596-018-0066-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0066-x
This article is cited by
-
Generation of iPSC-derived human forebrain organoids assembling bilateral eye primordia
Nature Protocols (2023)
-
Propofol Suppresses LPS-induced BBB Damage by Regulating miR-130a-5p/ZO-1 Axis
Molecular Biotechnology (2023)
-
Extracellular vesicles through the blood–brain barrier: a review
Fluids and Barriers of the CNS (2022)
-
Human mini-blood–brain barrier models for biomedical neuroscience research: a review
Biomaterials Research (2022)
-
Human organoids in basic research and clinical applications
Signal Transduction and Targeted Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.