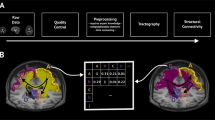
Abstract
Participant motion during functional magnetic resonance image (fMRI) acquisition produces spurious signal fluctuations that can confound measures of functional connectivity. Without mitigation, motion artifact can bias statistical inferences about relationships between connectivity and individual differences. To counteract motion artifact, this protocol describes the implementation of a validated, high-performance denoising strategy that combines a set of model features, including physiological signals, motion estimates, and mathematical expansions, to target both widespread and focal effects of subject movement. This protocol can be used to reduce motion-related variance to near zero in studies of functional connectivity, providing up to a 100-fold improvement over minimal-processing approaches in large datasets. Image denoising requires 40 min to 4 h of computing per image, depending on model specifications and data dimensionality. The protocol additionally includes instructions for assessing the performance of a denoising strategy. Associated software implements all denoising and diagnostic procedures, using a combination of established image-processing libraries and the eXtensible Connectivity Pipeline (XCP) software.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Sample data and results for testing the denoising and benchmarking protocol are available publicly at FigShare under the MIT license (https://doi.org/10.6084/m9.figshare.6225968.v1).
References
Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995).
Biswal, B. B. et al. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA 107, 4734–4739 (2010).
Power, J. D. et al. Functional network organization of the human brain. Neuron 72, 665–678 (2011).
Yeo, B. T. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl. Acad. Sci. USA 98, 676–682 (2001).
Sharma, A. et al. Common dimensional reward deficits across mood and psychotic disorders: a connectome-wide association study. Am. J. Psychiatry 174, 657–666 (2017).
Gu, S. et al. Emergence of system roles in normative neurodevelopment. Proc. Natl. Acad. Sci. USA 112, 13681–13686 (2015).
Dosenbach, N. U. F. et al. Prediction of individual brain maturity using fMRI. Science 329, 1358–1361 (2011).
Fair, D. A. et al. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. USA 105, 4028–4032 (2008).
Satterthwaite, T. D. et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage 60, 623–632 (2012).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Van Dijk, K. R. A., Sabuncu, M. R. & Buckner, R. L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438 (2012).
Power, J. D., Plitt, M., Laumann, T. O. & Martin, A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage 146, 609–625 (2016).
Fair, D. A. et al. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. USA 104, 13507–13512 (2007).
Friston, K., Williams, S., Howard, R., Frackowiak, R. S. J. & Turner, R. Movement-related effects in {fMRI} time-series. Magn. Reson. Med. 35, 346–355 (1996).
Behzadi, Y., Restom, K., Liau, J. & Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101 (2007).
Satterthwaite, T. D. et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64, 240–256 (2013).
Power, J. D. et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341 (2014).
Muschelli, J. et al. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 96, 22–35 (2014).
Pruim, R. H. R. et al. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277 (2015).
Salimi-Khorshidi, G. et al. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90, 449–468 (2014).
Ciric, R. et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage 154, 174–187 (2017).
Parkes, L., Fulcher, B. D., Yucel, M. & Fornito, A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage 171, 414–436 (2018).
Burgess, G. C. et al. Evaluation of denoising strategies to address motion-correlated magnetic resonance imaging data from the Human Connectome Project. Brain Connect. 6, 414–436 (2016).
Yan, C. G. et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76, 183–201 (2013).
Macey, P. M., Macey, K. E., Kumar, R. & Harper, R. M. A method for removal of global effects from fMRI time series. Neuroimage 22, 360–366 (2004).
Fox, M. D., Zhang, D., Snyder, A. Z. & Raichle, M. E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 101, 3270–3283 (2009).
Power, J. D., Schlaggar, B. L. & Petersen, S. E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105, 536–551 (2015).
Greene, D. J. et al. Behavioral interventions for reducing head motion during MRI scans in children. Neuroimage 171, 234–245 (2018).
Faraji-Dana, Z., Tam, F., Chen, J. J. & Graham, S. J. A robust method for suppressing motion-induced coil sensitivity variations during prospective correction of head motion in fMRI. Magn. Reson. Imaging 34, 1206–1219 (2016).
Bright, M. G. & Murphy, K. Removing motion and physiological artifacts from intrinsic BOLD fluctuations using short echo data. Neuroimage 64, 526–537 (2013).
Kundu, P. et al. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc. Natl. Acad. Sci. USA 110, 16187–16192 (2013).
Dosenbach, N. U. F. et al. Real-time motion analytics during brain MRI improve data quality and reduce costs. Neuroimage 161, 80–93 (2017).
Power, J. D. et al. Ridding fMRI data of motion-related influences: removal of signals with distinct spatial and physical bases in multiecho data. Proc. Natl. Acad. Sci. USA 115, E2105–E2114 (2018).
Satterthwaite, T. D. et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage 83, 45–57 (2013).
Zeng, L.-L. et al. Neurobiological basis of head motion in brain imaging. Proc. Natl. Acad. Sci. USA 111, 6058–6062 (2014).
Van Essen, D. C. et al. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79 (2013).
Satterthwaite, T. D. et al. Linked sex differences in cognition and functional connectivity in youth. Cereb. Cortex 25, 2383–2394 (2015).
Xia, C. H. et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat. Commun. 9, 3003 (2018).
Murphy, K. & Fox, M. D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154, 169–173 (2017).
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B. & Bandettini, P. A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905 (2009).
Chai, X. J., Castañán, A. N., Öngür, D. & Whitfield-Gabrieli, S. Anticorrelations in resting state networks without global signal regression. Neuroimage 59, 1420–1428 (2012).
Saad, Z. S. et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2, 25–32 (2012).
Yan, C.-G., Craddock, R. C., He, Y. & Milham, M. P. Addressing head motion dependencies for small-world topologies in functional connectomics. Front. Hum. Neurosci. 7, 910 (2013).
Power, J. D., Laumann, T. O., Plitt, M., Martin, A. & Petersen, S. E. On global fMRI signals and simulations. Trends Cogn. Sci. 21, 911–913 (2017).
Yang, G. J. et al. Altered global brain signal in schizophrenia. Proc. Natl. Acad. Sci. USA 111, 7438–7443 (2014).
Hahamy, A. et al. Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect. 4, 395–403 (2014).
Carone, D. et al. Impact of automated ICA-based denoising of fMRI data in acute stroke patients. NeuroImage Clin. 16, 23–31 (2017).
Tremblay, M., Tam, F. & Graham, S. J. Retrospective coregistration of functional magnetic resonance imaging data using external monitoring. Magn. Reson. Med. 53, 141–149 (2005).
Cox, R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, 208–219 (2004).
Lemieux, L., Salek-Haddadi, A., Lund, T. E., Laufs, H. & Carmichael, D. Modelling large motion events in fMRI studies of patients with epilepsy. Magn. Reson. Imaging 25, 894–901 (2007).
Klein, A. et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46, 786–802 (2009).
Tustison, N. J. et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage 99, 166–179 (2014).
Satterthwaite, T. D. et al. Motion artifact in studies of functional connectivity: characteristics and mitigation strategies. Hum. Brain Mapp. https://doi.org/10.1002/hbm.23665 (2017).
Power, J. D. A simple but useful way to assess fMRI scan qualities. Neuroimage 154, 150–158 (2017).
Zuo, X.-N. et al. An open science resource for establishing reliability and reproducibility in functional connectomics. Sci. Data 1, 140049 (2014).
Carp, J. Optimizing the order of operations for movement scrubbing: comment on Power et al. Neuroimage 76, 436–438 (2013).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage 76, 439–441 (2013).
Sikka, S. et al. Towards automated analysis of connectomes: the configurable pipeline for the analysis of connectomes (C-PAC). Abstract presented at Neuroinformatics 2013; 27–29 August 2013; Karolinska Institute, Stockholm. https://doi.org/10.3389/conf.fninf.2014.08.00117 (2012).
Shehzad, Z. et al. The preprocessed connectomes project quality assessment protocol—a resource for measuring the quality of MRI data. Abstract presented at Neuroinformatics 2015; 20–22 August 2015; Cairns, Australia. https://doi.org/10.3389/conf.fnins.2015.91.00047 (2015).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141 (2012).
Zuo, X. N. et al. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage 65, 374–386 (2013).
Esteban, O. et al. FMRIPrep: a robust preprocessing pipeline for functional MRI. Preprint at https://www.biorxiv.org/content/early/2018/07/24/306951 (2018).
Rosen, A. et al. Quantitative assessment of structural image quality. Neuroimage 169, 407–418 (2018).
Gordon, E. M. et al. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex 26, 288–303 (2016).
Cammoun, L. et al. Mapping the human connectome at multiple scales with diffusion spectrum MRI. J. Neurosci. Methods 203, 386–397 (2012).
Honnorat, N. et al. GraSP: Geodesic Graph-based Segmentation with Shape Priors for the functional parcellation of the cortex. Neuroimage 106, 207–221 (2015).
Schaefer, A. et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 1–20. https://doi.org/10.1093/cercor/bhx179 (2017).
Gorgolewski, K. J. et al. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci. Data 3, 1–9 (2016).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Power, J. D., Plitt, M., Kundu, P., Bandettini, P. A. & Martin, A. Temporal interpolation alters motion in fMRI scans: magnitudes and consequences for artifact detection. PLoS ONE 12, 1–20 (2017).
Nichols, T. E. Notes on creating a standardized version of DVARS. Preprint at https://arxiv.org/abs/1704.01469 (2017).
Afyouni, S. & Nichols, T. E. Insight and inference for DVARS. Neuroimage 172, 291–312 (2018).
Patel, A. X. et al. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage 95, 287–304 (2014).
Greve, D. N. & Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72 (2009).
Jo, H. J. et al. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. J. Appl. Math. https://doi.org/10.1155/2013/935154 (2013).
Cole, M. W., Bassett, D. S., Power, J. D., Braver, T. S. & Petersen, S. E. Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251 (2014).
Lomb, N. R. Least-squares frequency analysis of unequally spaced data. Astrophys. Space Sci. 39, 447–448 (1976).
Hallquist, M. N., Hwang, K. & Luna, B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 82, 208–225 (2013).
Smith, S. M. & Brady, J. M. SUSAN—a new approach to low level image processing. Int. J. Comput. Vis. 23, 45–78 (1997).
Blondel, V. D., Guillaume, J.-L., Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, P1008 (2008).
Girvan, M. & Newman, M. E. J. Community structure in social and biological networks. Proc. Natl. Acad. Sci. USA 99, 7821–7826 (2002).
Zang, Y., Jiang, T., Lu, Y., He, Y. & Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400 (2004).
Yu-Feng, Z. et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91 (2007).
Satterthwaite, T. D. et al. Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage 86, 544–553 (2014).
Pruim, R. H. R., Mennes, M., Buitelaar, J. K. & Beckmann, C. F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage 112, 278–287 (2015).
Acknowledgements
This work was supported by grants from the National Institutes of Health: R01MH107703 (T.D.S.), R01MH112847 (T.D.S.), R21MH106799 (D.S.B. and T.D.S.), R01EB022573 (C.D.), and R01MH101111 (D.H.W.); and the Lifespan Brain Institute at Penn/CHOP. D.S.B. acknowledges support from the John D. and Catherine T. MacArthur Foundation, the Alfred P. Sloan Foundation, the Army Research Laboratory through contract number W911NF1020022, the Army Research Office through contract numbers W911NF1410679 and W911NF1610474, and the National Institute of Health (grants R01DC00920911, R01HD086888, R01MH107235, R01MH109520, and R01NS099348).The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies.
Author information
Authors and Affiliations
Contributions
T.D.S., D.H.W., and R.C. developed and designed the protocol with numerous contributions from the functional neuroimaging community. R.C. developed the software implementation of the protocol. R.C., A.F.G.R., M.C., and A.A. developed the associated software libraries. M.C. and A.A. built Docker and Singularity containers for the associated software libraries. G.E. reviewed and tested the code and implemented the software on the Image Processing Portal. G.E., P.A.C., D.S.B., and C.D. provided consultation and guidance for methodological implementation and interpretation of results. R.C. and T.D.S. wrote the manuscript. R.C. prepared the figures and tables. All authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Satterthwaite, T. D. et al. Neuroimage 64, 240–256 (2013): https://doi.org/10.1016/j.neuroimage.2012.08.052
Ciric, R. et al. Neuroimage 154, 174–187 (2017): https://doi.org/10.1016/j.neuroimage.2017.03.020
Satterthwaite, T. D. et al. Neuroimage 83, 45–57 (2013): https://doi.org/10.1016/j.neuroimage.2013.06.045
Rights and permissions
About this article
Cite this article
Ciric, R., Rosen, A.F.G., Erus, G. et al. Mitigating head motion artifact in functional connectivity MRI. Nat Protoc 13, 2801–2826 (2018). https://doi.org/10.1038/s41596-018-0065-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0065-y
This article is cited by
-
Functional and structural reorganization in brain tumors: a machine learning approach using desynchronized functional oscillations
Communications Biology (2024)
-
A survey on Motion Artifact Correction in Magnetic Resonance Imaging for Improved Diagnostics
SN Computer Science (2024)
-
Alprazolam modulates persistence energy during emotion processing in first-degree relatives of individuals with schizophrenia: a network control study
Molecular Psychiatry (2023)
-
Intracranial electrophysiological and structural basis of BOLD functional connectivity in human brain white matter
Nature Communications (2023)
-
Evidence from “big data” for the default-mode hypothesis of ADHD: a mega-analysis of multiple large samples
Neuropsychopharmacology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.