Abstract
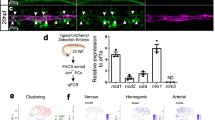
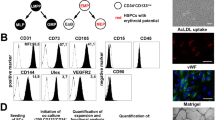
The ability to generate hematopoietic stem cells (HSCs) in vitro would have an immeasurable impact on many areas of clinical practice, including trauma, cancer, and congenital disease. In this protocol, we describe a stepwise approach that converts adult murine endothelial cells (ECs) to HSCs, termed ‘reprogrammed ECs into hematopoietic stem and progenitor cells’ (rEC-HSPCs). The conversion, which is achieved without cells transitioning through a pluripotent state, comprises three phases: induction, specification, and expansion. Adult ECs are first isolated from Runx1-IRES-GFP; Rosa26-rtTa mice and maintained in culture under EC growth factor stimulation and Tgfβ inhibition. In the first (induction) phase of conversion (days 0–8), four transcription factors (TFs)—FosB, Gfi1, Runx1, and Spi1 (FGRS)—are expressed transiently, which results in endogenous Runx1 expression. During the second (specification) phase (days 8–20), endogenous Runx1+ FGRS-transduced ECs commit to a hematopoietic fate and no longer require exogenous FGRS expression. Finally, the vascular niche drives robust proliferation of rEC-HSPCs during the expansion phase (days 20–28). The resulting converted cells possess a transcriptomic signature and long-term self-renewal capacity indistinguishable from those of adult HSCs. In this protocol, we also describe functional in vitro and in vivo assays that can be used to demonstrate that rEC-HSPCs are competent for clonal engraftment and possess multi-lineage reconstitution potential, including antigen-dependent adaptive immune function. This approach thus provides a tractable strategy for interrogating the generation of engraftable hematopoietic cells, advancing the mechanistic understanding of hematopoietic development and HSC self-renewal.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wahlster, L. & Daley, G. Q. Progress towards generation of human haematopoietic stem cells. Nat. Cell Biol. 18, 1111–1117 (2016).
de Bruijn, M. F., Speck, N. A., Peeters, M. C. & Dzierzak, E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 19, 2465–2474 (2000).
Heissig, B. et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109, 625–637 (2002).
Hooper, A. T. et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274 (2009).
Butler, J. M. et al. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood 120, 1344–1347 (2012).
Butler, J. M. et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264 (2010).
Poulos, M. G. et al. Vascular platform to define hematopoietic stem cell factors and enhance regenerative hematopoiesis. Stem Cell Rep. 5, 881–894 (2015).
Poulos, M. G. et al. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J. Clin. Invest. 127, 4163–4178 (2017).
Lis, R. et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature 545, 439–445 (2017).
Sandler, V. M., Lis, R. & Rafii, S. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature 511, 312–318 (2014).
Kobayashi, H. et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 12, 1046–1056 (2010).
Anderson, M. K., Weiss, A. H., Hernandez-Hoyos, G., Dionne, C. J. & Rothenberg, E. V. Constitutive expression of PU.1 in fetal hematopoietic progenitors blocks T cell development at the pro-T cell stage. Immunity 16, 285–296 (2002).
Batta, K., Florkowska, M., Kouskoff, V. & Lacaud, G. Direct reprogramming of murine fibroblasts to hematopoietic progenitor cells. Cell Rep. 9, 1871–1884 (2014).
Doulatov, S. et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell 13, 459–470 (2013).
Elcheva, I. et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat. Commun. 5, 4372 (2014).
Pereira, C. F. et al. Induction of a hemogenic program in mouse fibroblasts. Cell Stem Cell 13, 205–218 (2013).
Sturgeon, C. M., Ditadi, A., Clarke, R. L. & Keller, G. Defining the path to hematopoietic stem cells. Nat. Biotechnol. 31, 416–418 (2013).
Rafii, S. et al. Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood 121, 770–780 (2013).
Sugimura, R. et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 545, 432–438 (2017).
McLaughlin, F. et al. Combined genomic and antisense analysis reveals that the transcription factor Erg is implicated in endothelial cell differentiation. Blood 98, 3332–3339 (2001).
Riddell, J. et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell 157, 549–564 (2014).
Ginsberg, M., Schachterle, W., Shido, K. & Rafii, S. Direct conversion of human amniotic cells into endothelial cells without transitioning through a pluripotent state. Nat. Protoc. 10, 1975–1985 (2015).
Rafii, S. et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood 86, 3353–3363 (1995).
Rafii, S. et al. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood 84, 10–19 (1994).
Seandel, M. et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc. Natl Acad. Sci. USA 105, 19288–19293 (2008).
Acknowledgements
We thank J. Downing (St. Jude Hospital) for help in providing materials and for intellectual input. This work was supported by the Ansary Stem Cell Institute, the Starr Foundation Tri-Institutional Stem Cell Initiative Stem Cell Derivation Laboratory Core (Tri-SCI), and grants from the Daedalus Fund for Innovation of Weill Cornell Medicine (Daedalus); the Empire State Stem Cell Board of the New York State Department of Health (NYSTEM C029156); the US National Institutes of Health (NIH); the National Heart, Lung, and Blood Institute (NHLBI; R01HL128158, R01HL139056-01A1, and U01AI138329); the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK RC2DK114777); and the Qatar National Priorities Research Program (NPRP; 8-1898-3-392). J.G.B.D. was supported by the Ansary Stem Cell Institute and NIH/NHLBI R01HL139056-01A1. R.L. was supported by the Ansary Stem Cell Institute, Tri-SCI, NYSTEM C029156, NIH/NHLBI R01HL139056-01A1, NPRP 8-1898-3-392, and a NYSCF Druckenmiller Fellowship D-F56. T.M.L. was supported by Tri-SCI and the Avalon Fund. S.R. was supported by the Ansary Stem Cell Institute, Tri-SCI, Daedalus, NYSTEM C029156, NIH/NHLBI (R01HL128158, R01HL139056-01A1, and U01AI138329), NIH/NIDDK RC2DK114777, and NPRP 8-1898-3-392.
Author information
Authors and Affiliations
Contributions
S.R. envisioned the original idea. J.G.B.D. and R.L. developed the protocol and wrote the manuscript. J.G.B.D. performed the majority of the experiments. T.M.L. contributed to these experiments. R.L. conceived the project and interpreted the data. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.R. is the founder of and a nonpaid consultant to Angiocrine Bioscience, New York, NY, USA. The remaining authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Lis, R. et al. Nature 545, 439–445 (2017): https://doi.org/10.1038/nature22326
Sandler, V. M. et al. Nature 511, 312–318 (2014): https://doi.org/10.1038/nature13547
Seandel, M. et al. Proc. Natl Acad. Sci. USA 105, 19288–19293 (2008): https://doi.org/10.1073/pnas.0805980105
Rights and permissions
About this article
Cite this article
Barcia Durán, J.G., Lis, R., Lu, T.M. et al. In vitro conversion of adult murine endothelial cells to hematopoietic stem cells. Nat Protoc 13, 2758–2780 (2018). https://doi.org/10.1038/s41596-018-0060-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0060-3
This article is cited by
-
Endothelial Jak3 expression enhances pro-hematopoietic angiocrine function in mice
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.