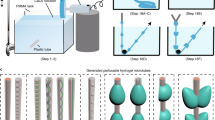
Abstract
This protocol describes the design of capillary microfluidics for spinning bioactive (cell-laden) microfibers for three-dimensional (3D) cell culture and tissue-engineering applications. We describe the assembly of three types of microfluidic systems: (i) simple injection capillary microfluidics for the spinning of uniform microfibers; (ii) hierarchical injection capillary microfluidics for the spinning of core–shell or spindle-knot structured microfibers; and (iii) multi-barrel injection capillary microfluidics for the spinning of microfibers with multiple components. The diverse morphologies of these bioactive microfibers can be further assembled into higher-order structures that are similar to the hierarchical structures in tissues. Thus, by using different types of capillary microfluidic devices, diverse styles of microfibers with different bioactive encapsulation can be generated. These bioactive microfibers have potential applications in 3D cell culture, the mimicking of vascular structures, the creation of synthetic tissues, and so on. The whole protocol for device fabrication and microfiber spinning takes ~1 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Langer, R. & Vacanti, J. P. Tissue engineering. Science 260, 920–926 (1993).
Place, E. S., George, J. H., Williams, C. K. & Stevens, M. M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 38, 1139–1151 (2009).
Sivagnanam, V. & Gijs, M. A. Exploring living multicellular organisms, organs, and tissues using microfluidic systems. Chem. Rev. 113, 3214–3247 (2013).
Huh, D., Hamilton, G. A. & Ingber, D. E. From 3D cell culture to organs-on-chips. Trends Cell Bio. 21, 745–754 (2011).
Kolesky, D. B., Homan, K. A., Skylar-Scott, M. A. & Lewis, J. A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 113, 3179–3184 (2016).
Yamada, K. M. & Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601–610 (2007).
Pampaloni, F., Reynaud, E. G. & Stelzer, E. H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 8, 839–845 (2007).
Zheng, F. et al. Organ-on-a-chip systems: microengineering to biomimic living systems. Small 12, 2253–2282 (2016).
Morimoto, Y. & Takeuchi, S. Three-dimensional cell culture based on microfluidic techniques to mimic living tissues. Biomater. Sci. 1, 257–264 (2013).
Derby, B. Printing and prototyping of tissues and scaffolds. Science 338, 921–926 (2012).
Pati, F., Gantelius, J. & Svahn, H. A. 3D bioprinting of tissue/organ models. Angew. Chem. Int. Ed. Engl. 55, 4650–4665 (2016).
Wang, H. & Heilshorn, S. C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 27, 3717–3736 (2015).
Wang, J. et al. Microfluidic generation of porous microcarriers for three-dimensional cell culture. ACS Appl. Mater. Interfaces 7, 27035–27039 (2015).
Yang, J. et al. Biomimetic nanofibers can construct effective tissue-engineered intervertebral discs for therapeutic implantation. Nanoscale 9, 13095–13103 (2017).
Leong, M. F. et al. Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres. Nat. Commun. 4, 2353 (2013).
Arrigoni, C. et al. Rational design of prevascularized large 3D tissue constructs using computational simulations and biofabrication of geometrically controlled microvessels. Adv. Healthc. Mater. 5, 1617–1626 (2016).
Wang, N. et al. A strategy for rapid and facile fabrication of controlled, layered blood vessel-like structures. RSC Adv. 6, 55054–55063 (2016).
Headen, D. M., Aubry, G., Lu, H. & García, A. J. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv. Mater. 26, 3003–3008 (2014).
Chung, B. G., Lee, K. H., Khademhosseinicdef, A. & Lee, S. H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip 12, 45–59 (2012).
Yuan, B. et al. A strategy for depositing different types of cells in three dimensions to mimic tubular structures in tissues. Adv. Mater. 24, 890–896 (2012).
Onoe, H. et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 12, 584–590 (2013).
Wu, F. et al. A novel synthetic microfiber with controllable size for cell encapsulation and culture. J. Mater. Chem. B 4, 2455–2465 (2016).
Onoe, H. & Takeuchi, S. Cell-laden microfibers for bottom-up tissue engineering. Drug Discov. Today 20, 236–246 (2015).
Cheng, Y. et al. Controlled fabrication of bioactive microfibers for creating tissue constructs using microfluidic techniques. ACS Appl. Mater. Interfaces 8, 1080–1086 (2016).
Shabahang, S. et al. Controlled fragmentation of multimaterial fibres and films via polymer cold-drawing. Nature 534, 529–533 (2016).
Zhang, C. L. & Yu, S. H. Spraying functional fibres by electrospinning. Mater. Horiz. 3, 266–269 (2016).
Liu, Y., Rafailovich, M. H., Malal, R., Cohn, D. & Chidambaram, D. Engineering of bio-hybrid materials by electrospinning polymer-microbe fibers. Proc. Natl. Acad. Sci. 106, 14201–14206 (2009).
Khalid, B. et al. Direct blow-spinning of nanofibers on a window screen for highly efficient PM2. 5 removal. Nano Lett. 17, 1140–1148 (2017).
Cheng, J., Jun, Y., Qin, J. & Lee, S. H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 114, 121–143 (2017).
Yoo, I., Song, S., Uh, K., Lee, C. W. & Kim, J. M. Size-controlled fabrication of polyaniline microfibers based on 3D hydrodynamic focusing approach. Macromol. Rapid Commun. 36, 1272–1276 (2015).
Daniele, M. A., Boyd, D. A., Adams, A. A. & Ligler, F. S. Microfluidic strategies for design and assembly of microfibers and nanofibers with tissue engineering and regenerative medicine applications. Adv. Healthc. Mater. 4, 11–28 (2015).
Jun, Y., Kang, E., Chae, S. & Lee, S. H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab Chip 14, 2145–2160 (2014).
Shang, L. et al. Bioinspired multifunctional spindle-knotted microfibers from microfluidics. Small 13, 1600286 (2017).
Håti, A. G. et al. Versatile, cell and chip friendly method to gel alginate in microfluidic devices. Lab Chip 16, 3718–3727 (2016).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Atencia, J. & Beebe, D. J. Controlled microfluidic interfaces. Nature 437, 648–655 (2004).
Shang, L., Cheng, Y. & Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 117, 7964–8040 (2017).
Nunes, J. K., Tsai, S. S. H., Wan, J. & Stone, H. A. Dripping and jetting in microfluidic multiphase flows applied to particle and fibre synthesis. J. Phys. D Appl. Phys. 46, 114002 (2013).
Aminian, M., Bernardi, F., Camassa, R., Harris, D. M. & McLaughlin, R. M. How boundaries shape chemical delivery in microfluidics. Science 354, 1252–1256 (2016).
Yoon, D. H., Tanaka, D., Sekiguchi, T. & Shoji, S. Microfluidic stamping on sheath flow. Small 12, 3224–3228 (2016).
Zhu, P., Kong, T., Kang, Z., Tian, X. & Wang, L. Tip-multi-breaking in capillary microfluidic devices. Sci. Rep. 5, 11102 (2015).
Kim, S. H. & Weitz, D. A. One-step emulsification of multiple concentric shells with capillary microfluidic devices. Angew. Chem. 123, 8890–8893 (2011).
Song, Y., Sauret, A. & Cheung Shum, H. All-aqueous multiphase microfluidics. Biomicrofluidics 7, 061301 (2013).
Mak, S. Y., Chao, Y. & Shum, H. C. The dripping-to-jetting transition in a co-axial flow of aqueous two-phase systems with low interfacial tension. RSC Adv. 7, 3287–3292 (2017).
Guillot, P., Colin, A., Utada, A. S. & Ajdari, A. Stability of a jet in confined pressure-driven biphasic flows at low Reynolds numbers. Phys. Rev. Lett. 99, 104502 (2007).
Lu, M. et al. Microfluidic hydrodynamic focusing for synthesis of nanomaterials. Nano Today 11, 778–792 (2016).
Håkansson, K. M. et al. Hydrodynamic alignment and assembly of nanofibrils resulting in strong cellulose filaments. Nat. Commun. 5, 4018 (2014).
Cheng, Y. et al. Bioinspired multicompartmental microfibers from microfluidics. Adv. Mater. 26, 5184–5190 (2014).
Zheng, Y. et al. Directional water collection on wetted spider silk. Nature 463, 640–643 (2010).
Shang, L. et al. Double emulsions from a capillary array injection microfluidic device. Lab Chip 14, 3489–3493 (2014).
Yu, Y. et al. Bioinspired helical microfibers from microfluidics. Adv. Mater. 29, 1605765 (2017).
Xu, P. et al. Bioinspired microfibers with embedded perfusable helical channels. Adv. Mater. 29, 1701664 (2017).
Nge, P. N., Rogers, C. I. & Woolley, A. T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 113, 2550–2583 (2013).
Ren, K., Zhou, J. & Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 46, 2396–2406 (2013).
Wang, J. et al. Microfluidic generation of Buddha beads-like microcarriers for cell culture. Sci. China Mater. 60, 857–865 (2017).
Yu, Y. et al. Microfluidic lithography of bioinspired helical micromotors. Angew. Chem. 56, 12127–12131 (2017).
Tian, Y. et al. Large-scale water collection of bioinspired cavity-microfibers. Nat. Commun. 8, 1080 (2017).
Shang, L. et al. Bio-inspired stimuli-responsive graphene oxide fibers from microfluidics. J. Mater. Chem. A 5, 15026–15030 (2017).
Shin, S. J. et al. “On the fly” continuous generation of alginate fibers using a microfluidic device. Langmuir 23, 9104–9108 (2007).
Abgrall, P. & Gue, A. M. Lab-on-chip technologies: making a microfluidic network and coupling it into a complete microsystem-a review. J. Micromech. Microeng. 17, R15 (2007).
Iliescu, C., Taylor, H., Avram, M., Miao, J. & Franssila, S. A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics 6, 016505 (2012).
Kuo, J. S. & Chiu, D. T. Disposable microfluidic substrates: transitioning from the research laboratory into the clinic. Lab Chip 11, 2656–2665 (2011).
McDonald, J. C. et al. Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis 21, 27–40 (2000).
Unger, M. A., Chou, H. P., Thorsen, T., Scherer, A. & Quake, S. R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288, 113–116 (2000).
Yu, Y. et al. Simple spinning of heterogeneous hollow microfibers on chip. Adv. Mater. 28, 6649–6655 (2016).
Kang, E. et al. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater. 10, 877–883 (2011).
Siegel, A. C. et al. Cofabrication: a strategy for building multicomponent microsystems. Acc. Chem. Res. 43, 518–528 (2010).
Liu, C. Recent developments in polymer MEMS. Adv. Mater. 19, 3783–3790 (2007).
Sun, K., Wang, Z. & Jiang, X. Modular microfluidics for gradient generation. Lab Chip 8, 1536–1543 (2008).
Hu, M. et al. Hydrodynamic spinning of hydrogel fibers. Biomaterials 31, 863–869 (2010).
Au, A. K., Huynh, W., Horowitz, L. F. & Folch, A. 3D-printed microfluidics. Angew. Chem. Int. Ed. 55, 3862–3881 (2016).
Ho, C. M. B., Ng, S. H., Li, K. H. H. & Yoon, Y. J. 3D printed microfluidics for biological applications. Lab Chip 15, 3627–3637 (2015).
Sperling, L. E., Reis, K. P., Pranke, P. & Wendorff, J. H. Advantages and challenges offered by biofunctional core-shell fiber systems for tissue engineering and drug delivery. Drug Discov. Today 21, 1243–1256 (2016).
Xiong, B. et al. Recent developments in microfluidics for cell studies. Adv. Mater. 26, 5525–5532 (2014).
Hosseini, V. et al. Fiber-assisted molding (FAM) of surfaces with tunable curvature to guide cell alignment and complex tissue architecture. Small 10, 4851–4857 (2014).
Akbari, M. et al. Textile technologies and tissue engineering: a path toward organ weaving. Adv. Healthc. Mater. 5, 751–766 (2016).
Russell, P. Photonic crystal fibers. Science 299, 358–362 (2003).
Nichol, J. W. et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5534–5536 (2010).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFA0700404), the National Science Foundation of China (grants 51522302 and 21473029), the NSAF Foundation of China (grant U1530260), the Fundamental Research Funds for the Central Universities, the Scientific Research Foundation of Southeast University, and the Scientific Research Foundation of the Graduate School of Southeast University.
Author information
Authors and Affiliations
Contributions
Y.Z. conceived the idea and designed the experiment. Y.Y. carried out the experiments. Y.Y., L.S., and Y.Z. analyzed the data and wrote the paper. J.G. and J.W. contributed to scientific discussion of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
1. Onoe, H. et al. Nat. Mater. 12, 584–590 (2013): https://www.nature.com/articles/nmat3606
2. Cheng, Y. et al. Adv. Mater. 26, 5184–5190 (2014): https://onlinelibrary.wiley.com/doi/full/10.1002/adma.201400798
3. Cheng, Y. et al. ACS Appl. Mater. Interfaces 8, 1080–1086 (2016): https://pubs.acs.org/doi/abs/10.1021/acsami.5b11445
4. Wang, J. et al. Sci. China Mater. 60, 857–865 (2017): https://link.springer.com/article/10.1007/s40843-017-9081-5
Integrated supplementary information
Supplementary Figure 1 Schematic illustration and digital photographs of the tapered capillaries.
Schematic and digital photographs of the tapered capillary with a (a) tapered tip and (b) spindle tip.
Supplementary Figure 2 Close-up images of the capillary assembly procedure.
(a) Immobilize the square capillary on the glass slide; (b) Assemble the injection and collection capillaries inside the square capillary with epoxy resin; (c) Insert the spindle injection capillary inside the origin injection channel with epoxy resin; (d) Cut the needles with small crevices at the bottom, and make their sizes match with that of the capillaries; (e) Coat the bottom margin of the needles with a thin layer of epoxy resin, and stand them onto the junctions of the capillaries. The upper of the (a-c, e) images are the corresponding scheme illustrations.
Supplementary Figure 3 Images of the simple microfluidic device, the spinning process and the produced simple microfiber.
(a) Digital photograph of the simple microfluidic device; (b) The microstructure of the device during the simple microfiber spinning; (c) Digital image of the continuous simple microfiber in a vessel; (d) Microscopic image of the generated cylindrical microfiber. Adapted with permission from Cheng et al. Bioinspired multicompartmental microfibers from microfluidics. Adv. Mater. 26, 5184–5190 (copyright 2014 Wiley) (https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.201400798). The scale bar is 200 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3
Supplementary Video 1
Creating tapered capillaries
Rights and permissions
About this article
Cite this article
Yu, Y., Shang, L., Guo, J. et al. Design of capillary microfluidics for spinning cell-laden microfibers. Nat Protoc 13, 2557–2579 (2018). https://doi.org/10.1038/s41596-018-0051-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0051-4
This article is cited by
-
Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications
Military Medical Research (2023)
-
Bamboo-Inspired Gasotransmitter Microfibres for Wound Healing
Advanced Fiber Materials (2023)
-
A highly integrated lab-on-a-disc immunoturbidimetric assay from whole blood with on-chip calibration
Microfluidics and Nanofluidics (2022)
-
Microfluidics in cardiovascular disease research: state of the art and future outlook
Microsystems & Nanoengineering (2021)
-
Composable microfluidic spinning platforms for facile production of biomimetic perfusable hydrogel microtubes
Nature Protocols (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.