Abstract
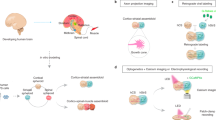
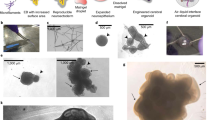
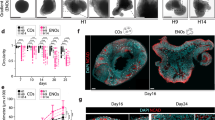
The ability to generate region-specific three-dimensional (3D) models to study human brain development offers great promise for understanding the nervous system in both healthy individuals and patients. In this protocol, we describe how to generate and assemble subdomain-specific forebrain spheroids, also known as brain region–specific organoids, from human pluripotent stem cells (hPSCs). We describe how to pattern the neural spheroids toward either a dorsal forebrain or a ventral forebrain fate, establishing human cortical spheroids (hCSs) and human subpallial spheroids (hSSs), respectively. We also describe how to combine the neural spheroids in vitro to assemble forebrain assembloids that recapitulate the interactions of glutamatergic and GABAergic neurons seen in vivo. Astrocytes are also present in the human forebrain–specific spheroids, and these undergo maturation when the forebrain spheroids are cultured long term. The initial generation of neural spheroids from hPSCs occurs in <1 week, with regional patterning occurring over the subsequent 5 weeks. After the maturation stage, brain region–specific spheroids are amenable to a variety of assays, including live-cell imaging, calcium dynamics, electrophysiology, cell purification, single-cell transcriptomics, and immunohistochemistry studies. Once generated, forebrain spheroids can also be matured for >24 months in culture.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pașca, S. P., Panagiotakos, G. & Dolmetsch, R. E. Generating human neurons in vitro and using them to understand neuropsychiatric disease. Annu. Rev. Neurosci. 37, 479–501 (2014).
Dolmetsch, R. & Geschwind, D. H. The human brain in a dish: the promise of iPSC-derived neurons. Cell 145, 831–834 (2011).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Brennand, K. J. & Gage, F. H. Modeling psychiatric disorders through reprogramming. Dis. Model. Mech. 5, 26–32 (2012).
Tabar, V. & Studer, L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat. Rev. Genet. 15, 82–92 (2014).
Brennand, K. J., Simone, A., Tran, N. & Gage, F. H. Modeling psychiatric disorders at the cellular and network levels. Mol. Psychiatry 17, 1239–1253 (2012).
Marchetto, M. C. et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 (2010).
Pașca, S. P. et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med. 17, 1657–1662 (2011).
Brennand, K. J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011).
Lee, G. et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406 (2009).
Wen, Z. et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 515, 414–418 (2014).
Kelava, I. & Lancaster, M. A. Stem cell models of human brain development. Cell Stem Cell 18, 736–748 (2016).
Di Lullo, E. & Kriegstein, A. R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 18, 573–584 (2017).
Pașca, S. P. The rise of three-dimensional human brain cultures. Nature 553, 437–445 (2018).
Pașca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Pașca, S. P. Personalized human cortical spheroids. Am. J. Psychiatry 173, 332–333 (2016).
Birey, F. et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59 (2017).
Sloan, S. A. et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790 (2017).
Deverman, B. E. et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 (2016).
Silbereis, J. C., Pochareddy, S., Zhu, Y., Li, M. & Sestan, N. The cellular and molecular landscapes of the developing human central nervous system. Neuron 89, 248–268 (2016).
Sousa, A. M. M., Meyer, K. A., Santpere, G., Gulden, F. O. & Sestan, N. Evolution of the human nervous system function, structure, and development. Cell 170, 226–247 (2017).
Stiles, J. & Jernigan, T. L. The basics of brain development. Neuropsychol. Rev. 20, 327–348 (2010).
Wonders, C. P. & Anderson, S. A. The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 7, 687–696 (2006).
Anderson, S. A., Marin, O., Horn, C., Jennings, K. & Rubenstein, J. L. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 128, 353–363 (2001).
Ma, T. et al. Subcortical origins of human and monkey neocortical interneurons. Nat. Neurosci. 16, 1588–1597 (2013).
Paredes, M. F. et al. Extensive migration of young neurons into the infant human frontal lobe. Science 354, https://doi.org/10.1126/science.aaf7073 (2016).
Kepecs, A. & Fishell, G. Interneuron cell types are fit to function. Nature 505, 318–326 (2014).
Marin, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 13, 107–120 (2012).
Rubenstein, J. L. & Merzenich, M. M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267 (2003).
Sloan, S. A. & Barres, B. A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 27C, 75–81 (2014).
Freeman, M. R. & Rowitch, D. H. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 80, 613–623 (2013).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Kadoshima, T. et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA 110, 20284–20289 (2013).
Mariani, J. et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375–390 (2015).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
Chambers, S. M., Mica, Y., Lee, G., Studer, L. & Tomishima, M. J. Dual-SMAD inhibition/WNT activation-based methods to induce neural crest and derivatives from human pluripotent stem cells. Methods Mol. Biol. https://doi.org/10.1007/7651_2013_59 (2013).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Muguruma, K., Nishiyama, A., Kawakami, H., Hashimoto, K. & Sasai, Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10, 537–550 (2015).
Camp, J. G. et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 112, 15672–15677 (2015).
Bagley, J. A., Reumann, D., Bian, S., Levi-Strauss, J. & Knoblich, J. A. Fused cerebral organoids model interactions between brain regions. Nat. Methods https://doi.org/10.1038/nmeth.4304 (2017).
Xiang, Y. et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383–398 (2017).
Quadrato, G., Brown, J. & Arlotta, P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med. 22, 1220–1228 (2016).
Tian, Y. et al. Alteration in basal and depolarization induced transcriptional network in iPSC derived neurons from Timothy syndrome. Genome Med. 6, 75 (2014).
Sun, Y. et al. A deleterious Nav1.1 mutation selectively impairs telencephalic inhibitory neurons derived from Dravet Syndrome patients. eLife https://doi.org/10.7554/eLife.13073 (2016).
Wang, X. & McManus, M. Lentivirus production. J. Vis. Exp. https://doi.org/10.3791/1499 (2009).
Acknowledgements
We thank the Pașca lab for experimental support. This work was supported by a US National Institute of Health (NIH) BRAINS Award (R01MH107800), the California Institute of Regenerative Medicine (CIRM), an MQ Fellow Award, and a Donald E. and Delia B. Baxter Foundation Award (to S.P.P.); NIMH T32GM007365, F30MH106261, and a Bio-X Predoctoral Fellowship (to S.A.S.); a Physician Scientist Development in Pediatrics Award (PSDP) and a Child Health Research Institute Postdoctoral Fellowship (to A.M.P.); Child Health Research Institute Postdoctoral Fellowship UL1-TR001085 (to F.B.); a Walter V. and Idun Berry Postdoctoral Fellowship (to J.A.); and Stanford Medicine’s Dean’s Fellowships (to F.B. and J.A.).
Author information
Authors and Affiliations
Contributions
S.A.S., J.A., A.M.P., and F.B. collected data and contributed to the optimizations of the protocols. S.A.S. and S.P.P. wrote the manuscript with input from all authors. S.P.P. supervised this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related Links
Key references using this protocol
Sloan, S. A. et al. Neuron 95, 779–790. (2017) https://doi.org/10.1016/j.neuron.2017.07.035
Birey, F. et al. Nature 545, 54–59 (2017) https://doi.org/10.1038/nature22330
Pașca, A. M. et al. Nat. Methods 12, 671–678 (2015) https://doi.org/10.1038/nmeth.3415
Rights and permissions
About this article
Cite this article
Sloan, S.A., Andersen, J., Pașca, A.M. et al. Generation and assembly of human brain region–specific three-dimensional cultures. Nat Protoc 13, 2062–2085 (2018). https://doi.org/10.1038/s41596-018-0032-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0032-7
This article is cited by
-
Modelling the complex nature of the tumor microenvironment: 3D tumor spheroids as an evolving tool
Journal of Biomedical Science (2024)
-
A patient-specific lung cancer assembloid model with heterogeneous tumor microenvironments
Nature Communications (2024)
-
Gliomas: a reflection of temporal gliogenic principles
Communications Biology (2024)
-
Kirigami electronics for long-term electrophysiological recording of human neural organoids and assembloids
Nature Biotechnology (2024)
-
iPSC-derived models of PACS1 syndrome reveal transcriptional and functional deficits in neuron activity
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.