Abstract
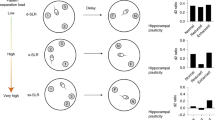
Pattern separation is the process of transforming highly similar sensory inputs into distinct, dissimilar representations. It takes place in the hippocampus and is thought to be used in episodic memory. Impaired pattern separation performance has been recognized as a predictor for the development of cognitive impairments such as dementia in humans and as being present in patients with schizophrenia and post-traumatic stress disorder (PTSD). In this protocol, we describe how to implement a simple and robust object pattern separation (OPS) task in mice and rats that we have previously established and validated. This two-trial memory task uses specific object locations so differences in performance can be calibrated with the extent of object movement. Changes in performance are indicative of spatial pattern separation. In contrast to other pattern separation tasks, the OPS task allows detection of spatial pattern separation performance bidirectionally. Furthermore, the OPS task is cheaper and easier to use and interpret than other tasks that use more than two objects or that are touch-screen based. The entire protocol, from vivarium acclimatization to training of the animals, takes ~35–41 d. After successful training, the animals can be tested repeatedly, and three OPS experiments (n = 20–24 per experimental day) can be performed per week. A standard level of expertise in behavioral studies in rodents is sufficient to successfully integrate this paradigm into an existing rodent test battery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Clelland, C. et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 (2009).
Kheirbek, M. A., Klemenhagen, K. C., Sahay, A. & Hen, R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat. Neurosci. 15, 1613–1620 (2012).
Reagh, Z. M. et al. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus 24, 303–314 (2013).
Schreiber, R. & Newman-Tancredi, A. Improving cognition in schizophrenia with antipsychotics that elicit neurogenesis through 5-HT1A receptor activation. Neurobiol. Learn. Mem. 110, 72–80 (2014).
Das, T., Ivleva, E. L., Wagner, A. D., Stark, C. E. L. & Tamminga, C. A. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophr. Res. 159, 193–197 (2014).
Tamminga, C. A., Stan, A. D. & Wagner, A. D. The hippocampal formation in schizophrenia. Am. J. Psychiatry 167, 1178–1193 (2010).
Bekinschtein, P. et al. BDNF in the dentate gyrus is required for consolidation of ‘pattern-separated’ memories. Cell Rep. 5, 759–768 (2013).
Oomen, C. A. et al. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat. Protoc. 8, 2006–2021 (2013).
Sahay, A., Wilson, D. A. & Hen, R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588 (2011).
Lacy, J. W., Yassa, M. A., Stark, S. M., Muftuler, L. T. & Stark, C. E. L. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn. Mem. 18, 15–18 (2011).
van Goethem, N. P., Schreiber, R., Newman-Tancredi, A., Varney, M. & Prickaerts, J. Divergent effects of the “biased”, 5-HT1A receptor agonists F15599 and F13714 in a novel object pattern separation task. Br. J. Pharmacol. 172, 2532–2543 (2015).
Ennaceur, A. & Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 31, 47–59 (1988).
Leger, M. et al. Object recognition test in mice. Nat. Protoc. 8, 2531–2537 (2013).
Antunes, M. & Biala, G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process 13, 93–110 (2012).
van Hagen, B. T. J., van Goethem, N. P., Lagatta, D. C. & Prickaerts, J. The object pattern separation (OPS) task; a behavioral paradigm derived from the object recognition task. Behav. Brain Res. 285, 44–52 (2015).
van Goethem, N. P. et al. Object recognition testing: rodent species, strains, housing conditions, and estrous cycle. Behav. Brain Res. 232, 323–334 (2012).
Heyser, J. & Chemero, A. Novel object exploration in mice: not all objects are created equal. Behav. Process. 89, 232–238 (2012).
Prusky, G. T., Harker, K. T., Douglas, R. M. & Whishaw, I. Q. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav. Brain Res. 136, 339–348 (2002).
Roedel, A., Storch, C., Holsboer, F. & Ohl, F. Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Lab. Anim. 40, 371–381 (2006).
Akkerman, S. et al. PDE5 inhibition improves object memory in standard housed rats but not in rats housed in an enriched environment: implications for memory models? PLoS ONE 9, e111692 (2014).
Bartolomucci, A. et al. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Pyschoneuroendocrinology 28, 540–558 (2003).
Arndt, S. S. et al. Individual housing of mice – impact on behaviour and stress responses. Physiol. Behav. 97, 385–393 (2009).
Castelhano-Carlos, M. J. & Baumans, V. The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats. Lab. Anim. 43, 311–327 (2009).
Ameen-Ali, K. E., Eacott, M. J. & Easton, A. A new behavioural apparatus to reduce animal numbers in multiple types of spontaneous object recognition paradigms in rats. J. Neurosci. Methods 211, 66–76 (2012).
Ameen-Ali, K. E., Easton, A. & Eacott, M. J. Moving beyond standard procedures to assess spontaneous recognition memory. Neurosci. Biobehav. Rev. 53, 37–51 (2015).
Albasser, M. M. et al. New behavioral protocols to extend our knowledge of rodent object recognition memory. Learn. Mem. 17, 407–419 (2010).
Kinnavane, L., Albasser, M. M. & Aggleton, J. P. Advances in the behavioural testing and network imaging of rodent recognition memory. Behav. Brain Res. 285, 67–78 (2015).
Sorge, R. E. et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 11, 629–632 (2014).
Ennaceur, A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav. Brain Res. 215, 244–254 (2010).
Akkerman, S. et al. Methodological considerations on discrimination and exploration measures in object recognition. Behav. Brain Res. 232, 335–347 (2012).
Bruno, O. et al. GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non-emetic doses. Br. J. Pharmacol. 164, 2054–2063 (2011).
Vanmierlo, T. et al. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol. Aging 32, 1262–1272 (2009).
Zeef, D. H. et al. Memory deficits in the transgenic rat model of Huntington’s disease. Behav. Brain Res. 227, 194–198 (2012).
Rutten, K. et al. Automated scoring of novel object recognition in rats. J. Neurosci. Methods 171, 72–77 (2008).
Akkerman, S., Prickaerts, J., Steinbusch, H. W. M. & Blokland, A. Object recognition testing: statistical considerations. Behav. Brain Res. 232, 317–322 (2012).
Acknowledgements
N.P.v.G. is financially supported by Alzheimer Nederland (grant no. WE.03-2017-11).
Author information
Authors and Affiliations
Contributions
N.P.v.G. and J.P. designed the general OPS procedure for measuring pattern separation performance in rodents. B.T.J.v.H. and N.P.v.G. performed the experiments and analyzed the data. N.P.v.G. and B.T.J.v.H wrote the manuscript with input from J. P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
1. van Hagen, B. T. J. et al. Behav. Brain Res. 285, 44–52 (2015): https://doi.org/10.1016/j.bbr.2014.10.041
2. van Goethem, N. P. et al. Br. J. Pharmacol. 172, 2532–2543 (2015): https://doi.org/10.1111/bph.13071.
Electronic supplementary material
Supplementary Data
This Excel file shows scoring of the supplementary video files; output and calculations of the parameters are outlined in the troubleshooting table
Supplementary Video 1
An example of a T1 OPS trial of a mouse. Trial duration is 4 min starting immediately upon placement of the mouse in the testing arena. Position 1 is utilized. The same mouse undertaking the T2 trial is shown in Supplementary Video 2 and the scoring and analysis of this trial is shown in the Supplementary Data
Supplementary Video 2
An example of a T2 OPS trial of a mouse. Trial duration is 4 min starting immediately upon placement of the mouse in the testing arena. Position 4 is utilized as a novel object location (right object moved backward to position 4). The same mouse undertaking the T1 trial is shown in Supplementary Video 1 and the scoring and analysis of this trial is shown in Supplementary the Data
Rights and permissions
About this article
Cite this article
van Goethem, N.P., van Hagen, B.T.J. & Prickaerts, J. Assessing spatial pattern separation in rodents using the object pattern separation task. Nat Protoc 13, 1763–1792 (2018). https://doi.org/10.1038/s41596-018-0013-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0013-x
This article is cited by
-
Anatomical and molecular characterization of parvalbumin-cholecystokinin co-expressing inhibitory interneurons: implications for neuropsychiatric conditions
Molecular Psychiatry (2023)
-
How to account for hallucinations in the interpretation of the antidepressant effects of psychedelics: a translational framework
Psychopharmacology (2022)
-
The spontaneous location recognition task for assessing spatial pattern separation and memory across a delay in rats and mice
Nature Protocols (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.