Abstract
The synthesis of RNA–DNA primer by primosome requires coordination between primase and DNA polymerase α subunits, which is accompanied by unknown architectural rearrangements of multiple domains. Using cryogenic electron microscopy, we solved a 3.6 Å human primosome structure caught at an early stage of RNA primer elongation with deoxynucleotides. The structure confirms a long-standing role of primase large subunit and reveals new insights into how primosome is limited to synthesizing short RNA–DNA primers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The cryo-EM maps of the human primosome elongation complexes have been deposited to the Electron Microscopy Data Bank (EMDB) under the accession code EMD-27256 (EC-I) and EMD-27258 (EC-II). The corresponding atomic coordinates are deposited in the RSCB Protein Data Bank under the accession code 8D96 (EC-I) and 8D9D (EC-II). The coordinates for the initial models used are available in the PDB under accession codes 5EXR (human p49, p58 and p70) and 4QCL (p180 and template:primer). Source data are provided with this paper.
References
Baranovskiy, A. G. & Tahirov, T. H. Elaborated action of the human primosome. Genes 8, 62 (2017).
Pellegrini, L. The Pol alpha-primase complex. Subcell. Biochem 62, 157–169 (2012).
Lim, C. & Cech, T. R. Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization. Nat. Rev. Mol. Cell Biol. 22, 283–298 (2021).
Fan, X. & Price, C. M. Coordinate regulation of G- and C strand length during new telomere synthesis. Mol. Biol. Cell 8, 2145–2155 (1997).
Reveal, P. M., Henkels, K. M. & Turchi, J. J. Synthesis of the mammalian telomere lagging strand in vitro. J. Biol. Chem. 272, 11678–11681 (1997).
Mirman, Z. et al. 53BP1-RIF1-shieldin counteracts DSB resection through CST- and Polα-dependent fill-in. Nature 560, 112–116 (2018).
Starokadomskyy, P. et al. DNA polymerase-α regulates the activation of type I interferons through cytosolic RNA:DNA synthesis. Nat. Immunol. 17, 495–504 (2016).
Kilkenny, M. L. et al. Structural basis for the interaction of SARS-CoV-2 virulence factor nsp1 with DNA polymerase α-primase. Protein Sci. 31, 333–344 (2021).
Tang, L., Sheraz, M., McGrane, M., Chang, J. & Guo, J. T. DNA polymerase α is essential for intracellular amplification of hepatitis B virus covalently closed circular DNA. PLoS Pathog. 15, e1007742 (2019).
Han, T. et al. The antitumor toxin CD437 is a direct inhibitor of DNA polymerase alpha. Nat. Chem. Biol. 12, 511–515 (2016).
Doublie, S. & Zahn, K. E. Structural insights into eukaryotic DNA replication. Front. Microbiol. 5, 444 (2014).
Sauguet, L., Klinge, S., Perera, R. L., Maman, J. D. & Pellegrini, L. Shared active site architecture between the large subunit of eukaryotic primase and DNA photolyase. PLoS ONE 5, e10083 (2010).
Vaithiyalingam, S., Warren, E. M., Eichman, B. F. & Chazin, W. J. Insights into eukaryotic DNA priming from the structure and functional interactions of the 4Fe-4S cluster domain of human DNA primase. Proc. Natl Acad. Sci. USA 107, 13684–13689 (2010).
Agarkar, V. B., Babayeva, N. D., Pavlov, Y. I. & Tahirov, T. H. Crystal structure of the C-terminal domain of human DNA primase large subunit: implications for the mechanism of the primase-polymerase alpha switch. Cell Cycle 10, 926–931 (2011).
Kilkenny, M. L., Longo, M. A., Perera, R. L. & Pellegrini, L. Structures of human primase reveal design of nucleotide elongation site and mode of Pol alpha tethering. Proc. Natl Acad. Sci. USA 110, 15961–15966 (2013).
Perera, R. L. et al. Mechanism for priming DNA synthesis by yeast DNA polymerase alpha. eLife 2, e00482 (2013).
Baranovskiy, A. G. et al. Structural basis for inhibition of DNA replication by aphidicolin. Nucleic Acids Res. 42, 14013–14021 (2014).
Vaithiyalingam, S. et al. Insights into eukaryotic primer synthesis from structures of the p48 subunit of human DNA primase. J. Mol. Biol. 426, 558–569 (2014).
Baranovskiy, A. G. et al. Crystal structure of the human primase. J. Biol. Chem. 290, 5635–5646 (2015).
Baranovskiy, A. G. et al. Mechanism of concerted RNA–DNA primer synthesis by the human primosome. J. Biol. Chem. 291, 10006–10020 (2016).
Baranovskiy, A. G. et al. Activity and fidelity of human DNA polymerase alpha depend on primer structure. J. Biol. Chem. 293, 6824–6843 (2018).
Klinge, S., Nunez-Ramirez, R., Llorca, O. & Pellegrini, L. 3D architecture of DNA Pol alpha reveals the functional core of multi-subunit replicative polymerases. EMBO J. 28, 1978–1987 (2009).
Nunez-Ramirez, R. et al. Flexible tethering of primase and DNA Pol α in the eukaryotic primosome. Nucleic Acids Res. 39, 8187–8199 (2011).
Kilkenny, M. L. et al. Structural basis for the interaction of SARS-CoV-2 virulence factor nsp1 with DNA polymerase α-primase. Protein Sci. 31, 333–344 (2022).
He, Q. et al. Structures of the human CST–Polα–primase complex bound to telomere templates. Nature 608, 826–832 (2022).
Baranovskiy, A. G. et al. Insight into the human DNA primase interaction with template–primer. J. Biol. Chem. 291, 4793–4802 (2016).
Sheaff, R. J., Kuchta, R. D. & Ilsley, D. Calf thymus DNA polymerase α-primase: ‘communication’ and primer–template movement between the two active sites. Biochemistry 33, 2247–2254 (1994).
Arezi, B., Kirk, B. W., Copeland, W. C. & Kuchta, R. D. Interactions of DNA with human DNA primase monitored with photoactivatable cross-linking agents: implications for the role of the p58 subunit. Biochemistry 38, 12899–12907 (1999).
Baranovskiy, A. G., Lisova, A. E., Morstadt, L. M., Babayeva, N. D. & Tahirov, T. H. Insight into RNA–DNA primer length counting by human primosome. Nucleic Acids Res. 50, 6264–6270 (2022).
Reijns, M. A. M. et al. Lagging-strand replication shapes the mutational landscape of the genome. Nature 518, 502–506 (2015).
Baranovskiy, A. G. et al. Crystallization and preliminary X-ray diffraction analysis of human DNA primase. Acta Crystallogr. F 70, 206–210 (2014).
Zhang, Y., Baranovskiy, A. G., Tahirov, T. H. & Pavlov, Y. I. The C-terminal domain of the DNA polymerase catalytic subunit regulates the primase and polymerase activities of the human DNA polymerase α-primase complex. J. Biol. Chem. 289, 22021–22034 (2014).
Mizuno, T. et al. The intrinsically disordered N-terminal region of mouse DNA polymerase α mediates its interaction with POT1a/b at telomeres. Genes Cells 26, 360–380 (2021).
Liu, H. & Naismith, J. H. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 8, 91 (2008).
Noble, A. J. et al. Reducing effects of particle adsorption to the air–water interface in cryo-EM. Nat. Methods 15, 793–795 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Biol. Crystallogr. 74, 531–544 (2018).
Acknowledgements
We thank members of the Lim lab and Tahirov lab for their helpful discussions. We also thank M. Cox and I. Rayment for their constructive feedback. We also thank Z. Xu for her assistance in figures preparation. This research was, in part, supported by the Cryo-EM Research Center in the Department of Biochemistry at the University of Wisconsin-Madison and the National Cancer Institute’s National Cryo-EM Facility at the Frederick National Laboratory for Cancer Research under contract HSSN261200800001E. This work was supported by the National Institute of General Medical Sciences grants R35GM127085 to T.H.T. and R00GM131023 to C.L. Support for this work was also provided to C.L. by the University of Wisconsin–Madison, Office of the Vice-Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation and the Department of Biochemistry.
Author information
Authors and Affiliations
Contributions
Q.H. carried out cryo-EM sample preparation, data collection, map construction and structure refinement with supervision by C.L. Q.H. and C.L. designed the primase mutations. Q.H. and B.L.L. made the recombinant mutant primase proteins. A.G.B. developed the protocol for chimeric primer synthesis, carried out the biochemical experiments and supervised preparation of samples for structural and functional studies, with help from L.M.M., A.E.L. and N.D.B. T.H.T. initiated the project and participated in initial model building and refinement. Q.H. and C.L. wrote the manuscript with support from A.G.B. and T.H.T.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Sara Osman was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM processing pipeline for the elongation complexes.
(a) Representative micrograph (n = 13,243) of the cryo-EM dataset. The scale bar dimension is 500 Å. (b) 2D classification averages of the elongation complexes. (c) Cryo-EM processing pipeline that was used to obtain the two cryo-EM maps of elongation complexes I and II.
Extended Data Fig. 2 Local resolution maps and map to model comparison of the cryo-EM reconstruction.
Local resolution maps of the (a) EC-I and (b) EC-II cryo-EM structures. (c) Representative cryo-EM densities encasing the corresponding atomic models of DNA template, RNA-DNA primer, p49, p58 and p180.
Extended Data Fig. 3 Structural comparison of template:primer bound to Polα catalytic core and to p58C between the two cryo-EM elongation complex models.
(a) The models of elongation complex I and II are aligned and the RMSD values of p58C, p180core, RNA-DNA primer, and DNA template are calculated. Their RMSD values are shown in the table in the panel. A ribbon model of the EC-II is shown with its subunits colored as per described in main text. The superimposed EC-I ribbon model is colored grey to illustrate the similarity between the two models. The ChimeraX software37 was used to perform the above RMSD analysis. (b) The crystal structure of p180core/template:primer (PDB code: 4QCL) was superimposed with p180core/template:primer part of primosome elongation complex II (RMSD = 1.10 Å2). (c) The crystal structure of template:primer/p58C (PDB code: 5F0Q) was superimposed with template:primer/p58C part of primosome elongation complex II (RMSD = 0.59 Å2).
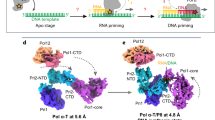
Extended Data Fig. 4 Primosome undergoes a large conformation change as it progresses from the apo to elongation state.
The apo state of human primosome (PDB code: 5EXR) is compared with this work’s elongation states primosome, (a) EC-I and (b) EC-II. The four subunits of APO primosome are colored: p49 as green, p58 as dark grey, p70 as olive green, and p180 as salmon red. The elongation state primosomes are colored as described in Fig. 1. The APO and EC-I/II structures are aligned using the p180 subunit. Visual inspection of APO vs EC-II state shows the p49, p58, and p70 domains are rearranged relative to the p180core domain.
Extended Data Fig. 5 Mutagenesis analysis of platform-p180core interaction by primer extension assay using primosome with full-length Polα.
(a) SDS-PAGE analysis of tandem affinity purified full-length Primosome. 6xHis-tagged subunits (p49, p58, p70) were first enriched before pulling down the Twin-Strep-tagged p180 to obtain the assembled primosome complex. FT: flowthrough. The results are reproduced across multiple independent experiments (n > 3). (b) Mutations disrupting the platform-p180core interaction increase processivity of DNA synthesis. The mutant primosome (Polα/p49K95E/L96A/p58R235E), with full-length p180, is referred as 3 m in the panels. T1:P2 was used in all reactions. (c) Processivity quantification of the results from panel a using fraction of longer products (defined as >37nt products for -trap and >33nt products for +trap) over total product made. Triplicate data are represented as hollow circles while their mean and standard deviation (mean ± SD) are show as columns and error bars, respectively.
Extended Data Fig. 6 Detailed mutagenesis analysis of the platform-p180core interaction.
(a) Purified recombinant wild-type and mutant human primosomes. Mutant primosomes are annotated in gel as 1 m (ΔN-Polα/p49/p58R235E), 2 m (ΔN-Polα/p58/p49K95E/L96A), and 3 m (ΔN-Polα/p49K95E/L96A/p58R235E). This experiment was performed once. (b) Mutation(s) on either p49 or p58 has a similar effect as the combined mutation (3 m) on enzyme processivity. T1:P2 was used in all reactions without trap added. (c) Processivity quantification using fraction of longer products (>37nt) over total product from triplicates of panel b results. The triplicate data are represented as hollow circles while their mean and standard deviation (mean ± SD) are shown as columns and error bars.
Extended Data Fig. 7 DNA synthesis by primosome upon disruption of the interaction between p58C and template:primer.
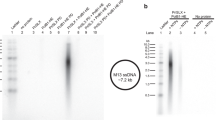
As compared to the RNA-DNA primers made by wild-type primosome using template:primer with 5’-triphosphate (see Fig. 2d, lane 1), reactions using primosome with deleted p58C or the template:primer without the 5’-triphosphate resulted in longer products being made by the enzyme. Reactions corresponding to the left and right lanes contain T1:P2 and T1-P1, respectively. The results are reproducible across multiple independent experiments (n = 3).
Supplementary information
Source data
Source Data Fig. 2
Unprocessed gel.
Source Data Extended Data Fig. 5
Unprocessed gels.
Source Data Extended Data Fig. 6
Unprocessed gels.
Source Data Extended Data Fig. 7
Unprocessed gel.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Q., Baranovskiy, A.G., Morstadt, L.M. et al. Structures of human primosome elongation complexes. Nat Struct Mol Biol 30, 579–583 (2023). https://doi.org/10.1038/s41594-023-00971-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-00971-3
This article is cited by
-
A mechanistic model of primer synthesis from catalytic structures of DNA polymerase α–primase
Nature Structural & Molecular Biology (2024)
-
Molecular choreography of primer synthesis by the eukaryotic Pol α-primase
Nature Communications (2023)