Abstract
The universally conserved enzyme phosphoribosyl pyrophosphate synthetase (PRPS) assembles filaments in evolutionarily diverse organisms. PRPS is a key regulator of nucleotide metabolism, and mutations in the human enzyme PRPS1 lead to a spectrum of diseases. Here we determine structures of human PRPS1 filaments in active and inhibited states, with fixed assembly contacts accommodating both conformations. The conserved assembly interface stabilizes the binding site for the essential activator phosphate, increasing activity in the filament. Some disease mutations alter assembly, supporting the link between filament stability and activity. Structures of active PRPS1 filaments turning over substrate also reveal coupling of catalysis in one active site with product release in an adjacent site. PRPS1 filaments therefore provide an additional layer of allosteric control, conserved throughout evolution, with likely impact on metabolic homeostasis. Stabilization of allosteric binding sites by polymerization adds to the growing diversity of assembly-based enzyme regulatory mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The cryo-EM maps generated for this manuscript are available from the EMDB (https://www.ebi.ac.uk/emdb/) at the accession codes listed in Tables 1–3 of the manuscript (EMDB IDs: EMD-27279, EMD-27280, EMD-27281, EMD-27282, EMD-27283, EMD-27284, EMD-27285, EMD-27286, EMD-27287, EMD-27288, EMD-27289, EMD-27290, EMD-27291, EMD-27292, EMD-27293, EMD-27294, EMD-27295). The protein models generated for this manuscript are available from the RCSB PDB (https://www.rcsb.org/) at the accession codes listed in Tables 1–3 of the manuscript (PDB IDs: 8DBC, 8DBD, 8DBE, 8DBF, 8DBG, 8DBH, 8DBI, 8DBJ, 8DBK, 8DBL, 8DBM, 8DBN, 8DBO). Protein sequences identified by the NCBI online portal for BLAST (v2.13.0; https://blast.ncbi.nlm.nih.gov/Blast.cgi) were queried on the ‘non-redundant protein sequences (nr)’ database. Source data are provided with this paper.
References
Hove-Jensen, B. et al. Phosphoribosyl diphosphate (PRPP): biosynthesis, enzymology, utilization and metabolic significance. Microbiol. Mol. Biol. Rev. 81, e00040-16 (2017).
Kornberg, A., Lieberman, I. & Simms, E. S. Enzymatic synthesis and properties of 5-phosphoribosylpyrophosphate. J. Biol. Chem. 215, 389–402 (1955).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Fagerberg, L. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 13, 397–406 (2014).
de Brouwer, A. P. M. et al. PRPS1 mutations: four distinct syndromes and potential treatment. Am. J. Hum. Genet. 86, 506–518 (2010).
Sperling, O., Eilam, G., And, S.-P.-B. & de Vries, A. A. Familial abnormality associated with excessive uric acid production and gout. Biochem. Med. 6, 310–316 (1972).
Wada, Y. et al. Mentally retarded infant with a defect of 5-phosphoribosyl-I-pyrophosphate synthetase of erythrocytes. Tohoku J. Exp. Med. 113, 149–157 (1974).
Willemoës, M., Hove-Jensen, B. & Larsen, S. Steady state kinetic model for the binding of substrates and allosteric effectors to Escherichia coli phosphoribosyl-diphosphate synthase. J. Biol. Chem. 275, 35408–35412 (2000).
Fox, I. H. & Kelley, W. N. Human phosphoribosylpyrophosphate synthetase: distribution, purification and properties. J. Biol. Chem. 246, 5739–5748 (1971).
Switzer, R. L. & Sogin, D. C. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. J. Biol. Chem. 248, 1063–1073 (1973).
Tatibana, M. et al. Mammalian phosphribosyl-pyrophosphate synthetase. Adv. Enzym. Regul. 35, 229–249 (1995).
Hernando, Y., Carter, A. T., Parr, A., Hove-Jensen, B. & Schweizer, M. Genetic analysis and enzyme activity suggest the existence of more than one minimal functional unit capable of synthesizing phosphoribosyl pyrophosphate in Saccharomyces cerevisiae. J. Biol. Chem. 274, 12480–12487 (1999).
Taira, M. et al. Nucleotide and deduced amino acid sequences of two distinct cDNAs for rat phosphoribosylpyrophosphate synthetase. J. Biol. Chem. 262, 14867–14870 (1987).
Taira, M., Lizasa, T., Yamada, K., Shimada, H. & Tatibana, M. Tissue-differential expression of two distinct genes for phosphoribosyl pyrophosphate synthetase and existence of the testis-specific transcript. Biochim. Biophys. Acta 1007, 203–208 (1989).
Ishizuka, T. et al. Short sequence-paper cloning and sequencing of human complementary DNA for the phosphoribosylpyrophosphate synthetase-associated protein 39. Biochim. Biophys. Acta 1306, 27–30 (1996).
Katashima, R. et al. Molecular cloning of a human cDNA for the 41-kDa phosphoribosylpyrophosphate synthetase-associated protein 1. Biochim. Biophys. Acta 1396, 245–250 (1998).
Kita, K., Ishizuka, T., Ishijima, S., Sonoda, T. & Tatibana, M. A novel 39-kDa phosphoribosylpyrophosphate synthetase-associated protein of rat liver. J. Biol. Chem. 269, 8334–8340 (1994).
Eriksen, T. A., Kadziola, A., Bentsen, A.-K., Harlow, K. W. & Larsen, S. Structural basis for the function of Bacillus subtilis phosphoribosyl-pyrophosphate synthetase. Nature 7, 303–308 (2000).
Li, S., Lu, Y., Peng, B. & Ding, J. Crystal structure of human phosphoribosylpyrophosphate synthetase 1 reveals a novel allosteric site. Biochem. J. 401, 39–47 (2007).
Roth, D. G., Shelton, E. & Deuel, T. F. Purification and properties of phosphoribosyl pyrophosphate synthetase from rat liver. J. Biol. Chem. 249, 291–296 (1974).
Becker, M. A., Meyer, L. J., Huisman, W. H., Lazar, C. & Adams, W. B. Human erythrocyte phosphoribosylpyrophosphate synthetase: subunit analysis and atates of subunit association. J. Biol. Chem. 252, 3911–3918 (1977).
Meyer, L. J. & Becker, M. A. Human erythrocyte phosphoribosylpyrophosphate synthetase: dependance of activity on state of subunit association. J. Biol. Chem. 252, 3919–3925 (1977).
Zerez, C. A., Lachant, N. A. & Tanaka, K. R. Decrease in subunit aggregation of phosphoribosylpyrophosphate synthetase: a mechanism for decreased nucleotide concentrations in pyruvate kinase-deficient human erythrocytes. Blood 68, 1024–1029 (1986).
Baugh, L. et al. Combining functional and structural genomics to sample the essential Burkholderia structome. PLoS ONE 8, e53851 (2013).
Donini, S. et al. Biochemical and structural investigations on phosphoribosylpyrophosphate synthetase from mycobacterium smegmatis. PLoS ONE 12, e0175815 (2017).
Timofeev, V. I. et al. Crystal structure of recombinant phosphoribosylpyrophosphate synthetase 2 from Thermus thermophilus HB27 complexed with ADP and sulfate ions. Acta Crystallogr. F Struct. Biol. Commun. 73, 369–375 (2017).
Hu, H. H. et al. Filamentation modulates allosteric regulation of PRPS. eLife 11, e79552 (2022).
Noree, C. et al. A quantitative screen for metabolic enzyme structures reveals patterns of assembly across the yeast metabolic network. Mol. Biol. Cell 30, 2721–2736 (2019).
Begovich, K., Yelon, D. & Wilhelm, J. E. PRPS polymerization influences lens fiber organization in zebrafish. Dev. Dyn. 249, 1018–1031 (2020).
Park, C. K. & Horton, N. C. Structures, functions and mechanisms of filament forming enzymes: a renaissance of enzyme filamentation. Biophys. Rev. 11, 927–994 (2019).
Lynch, E. M., Kollman, J. M. & Webb, B. A. Filament formation by metabolic enzymes—a new twist on regulation. Curr. Opin. Cell Biol. 66, 28–33 (2020).
Simonet, J. C., Burrell, A. L., Kollman, J. M. & Peterson, J. R. Freedom of assembly: metabolic enzymes come together. Mol. Biol. Cell 31, 1201–1205 (2020).
Lynch, E. M. & Kollman, J. M. Coupled structural transitions enable highly cooperative regulation of human CTPS2 filaments. Nat. Struct. Mol. Biol. 27, 42–48 (2020).
Hershko, A., Razin, A. & Mager, J. Regulation of the synthesis of 5-phosphoribosyl-1-pyrophosphate in intact red blood cells and in cell-free preparations. Biochim. Biophys. Acta 184, 64–76 (1969).
Losman, M. J. & Becker, M. A. Human phosphoribosyl pyrophosphate (PRPP) synthetase requirements for subunit aggregation. Adv. Exp. Med. Biol. 165, 427–432 (1984).
Burrell, A. L. et al. IMPDH1 retinal variants control filament architecture to tune allosteric regulation. Nat. Struct. Mol. Biol. 29, 47–58 (2022).
Johnson, M. C. & Kollman, J. M. Cryo-EM structures demonstrate human IMPDH2 filament assembly tunes allosteric regulation. Elife 9, e53243 (2020).
Hansen, J. M. et al. Cryo-EM structures of CTP synthase filaments reveal mechanism of pH-sensitive assembly during budding yeast starvation. eLife 10, e73368 (2021).
Zhou, W. et al. Crystal structure of E. coli PRPP synthetase. BMC Struct. Biol. 19, 1 (2019).
Chen, P. et al. Crystal and EM structures of human phosphoribosyl pyrophosphate synthase I (PRS1) provide novel insights into the disease-associated mutations. PLoS ONE 10, e0120304 (2015).
Cherney, M. M., Cherney, L. T., Garen, C. R. & James, M. N. G. The structures of Thermoplasma volcanium phosphoribosyl pyrophosphate synthetase bound to ribose-5-phosphate and ATP analogs. J. Mol. Biol. 413, 844–856 (2011).
Almoguera, B. et al. Expanding the phenotype of PRPS1 syndromes in females: neuropathy, hearing loss and retinopathy. Orphanet J. Rare Dis. 9, 190 (2014).
Liu, X. et al. Loss-of-function mutations in the PRPS1 gene cause a type of nonsyndromic X-linked sensorineural deafness, DFN2. Am. J. Hum. Genet. 86, 65–71 (2010).
Robusto, M. et al. The expanding spectrum of PRPS1-associated phenotypes: three novel mutations segregating with X-linked hearing loss and mild peripheral neuropathy. Eur. J. Hum. Genet. 23, 766–773 (2015).
Zoref, E., de Vries, A. & Sperling, O. Mutant feedback resistant phosphoribosylpyrophosphate synthetase associated with purine overproduction and gout. Phosphoribosylpyrophosphate and purine metabolism in cultured fibroblasts. J. Clin. Invest. 56, 1093–1099 (1975).
Becker, M. A., Smith, P. R., Taylor, W., Mustafi, R. & Switzer, R. L. The genetic and functional basis of purine nucleotide feedback-resistant phosphoribosylpyrophosphate synthetase superactivity. J. Clin. Invest. 96, 2133–2141 (1995).
Chen, P., Li, J., Ma, J., Teng, M. & Li, X. A small disturbance, but a serious disease: the possible mechanism of D52H-mutant of human PRS1 that causes gout. IUBMB Life 65, 518–525 (2013).
Sperling, O., Boer, P., Brosh, S., Zoref, E. & de Vries, A. Overproduction disease in man due to enzyme feedback resistance mutation. Enzyme 23, 1–9 (1978).
Cunningham, J. T., Moreno, M. V., Lodi, A., Ronen, S. M. & Ruggero, D. Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell 157, 1088–1103 (2014).
Lu, G. et al. Structural basis of human PRPS2 filaments. Preprint at https://www.biorxiv.org/content/10.1101/2022.07.11.499506v1 (2022).
Calise, S. J., Abboud, G., Kasahara, H., Morel, L. & Chan, E. K. L. Immune response-dependent assembly of IMP dehydrogenase filaments. Front. Immunol. 9, 2789 (2018).
Duong-Ly, K. C. et al. T cell activation triggers reversible inosine-5′-monophosphate dehydrogenase assembly. J. Cell Sci. 131, cs223289 (2018).
Zi Tan, Y. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Mossessova, E. & Lima, C. D. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol. Cell 5, 865–876 (2000).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. W. in Methods in Enzymology Vol. 579 (ed. Crowther, R. A.) 125–157 (Academic Press, 2016).
Terwilliger, T. C., Ludtke, S. J., Read, R. J., Adams, P. D. & Afonine, P. V. Improvement of cryo-EM maps by density modification. Nat. Methods 17, 923–927 (2020).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Pettersen, E. F. et al. UCSF Chimera – a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 74, 519–530 (2018).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Madeira, F. et al. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 50, W276–W279 (2022).
RStudio Team. RStudio: Integrated Development for R (2020); http://www.rstudio.com/
R Core Team. R: A Language and Environment for Statistical Computing (2022); https://www.R-project.org
Wickham, H., François, R., Henry, L. & Müller, K. dplyr: A Grammar of Data Manipulation (2022); https://dplyr.tidyverse.org, https://github.com/tidyverse/dplyr
Ritz, C., Baty, F., Streibig, J. C. & Gerhard, D. Dose-response analysis using R. PLoS ONE 10, e0146021 (2015).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (2016); https://ggplot2.tidyverse.org
Acknowledgements
We thank the Arnold and Mabel Beckman Cryo-EM Center at the University of Washington for electron microscope use. We also thank members of the Kollman group for valuable feedback provided during cryo-EM data collection and processing. This work was supported by the US National Institutes of Health (grants nos. R01GM118396 and S10OD023476 to J.M.K. and 1F32AI145111 to K.L.H.)
Author information
Authors and Affiliations
Contributions
K.L.H. performed experiments. K.H. optimized protein purification. J.D.Q. arranged, guided and provided support for EM data collection. K.L.H. and J.M.K. designed experiments, performed data analysis and interpretation, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Ambroise Desfosses, Arjen Jakobi and Menico Rizzi for their contribution to the peer review of this work. Primary Handling Editors: Florian Ullrich, Carolina Perdigoto and Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
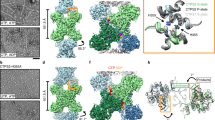
Extended Data Fig. 1 Filament formation in PRPS1.
a. Section of negative stain EM of purified PRPS protein in a HEPES/Salt buffer used for purification. b. Panel of negative stain EM sections of PRPS1 in phosphate buffer in the presence of the indicated ligands. c. Elution profile from a size exclusion column (Superose 6) of PRPS1 in 50 mM phosphate buffer, pH 7.6. d. Elution profile from a size exclusion column (Superose 6) of PRPS1 in 100 mM KCl, 50 mM HEPES, pH 7.6 in the presence (black) or absence (grey) of 50 mM potassium phosphate, pH 7.6. PRPS1: monomer, 35 kDa; dimer, 70 kDa; hexamer, 210 kDa; filament, ≧ 420 kDa. e. Motion-corrected and summed cryo electron micrographs, Gaussian blurred and contrast adjusted for visualization, from four datasets presented in this manuscript, representing the cameras and tilts used in data collection. Microscopes, cameras, and stage tilts are listed in Data Table 1.
Extended Data Fig. 2 Data processing and statistics for cryo-EM datasets.
Left. Overview of the processing scheme for the datasets presented in this manuscript. Right. Fourier shell correlation curves calculated in Relion (black) and in Phenix (grey). One set of curves per dataset is shown. For full dataset statistics and information, see Data Table 1.
Extended Data Fig. 3 Volumes and models of filament interface residues.
a. Surface representation of filament interface in phosphate- or ADP-bound structures; orange patches indicate residues involved in the interface. b. Model and map of the primary interface residues of filament structures presented in this manuscript (the interface from the ADP bound filament can be found in Fig. 2c). Right panel shows the overlay of the interfaces when aligned by the bottom protomer. ADP-bound structure colored in orange and blue; all others in grey. c. Schematic of primary interactions across the filament interface; rectangular dashed lines indicate pi-stacking interactions and rounded dashed lines indicate hydrogen bonds. d. The C-terminal portion of a protein sequence alignment comparing PRPS across kingdoms. Identical residues are highlighted in orange. e. Alignment of the phosphate and ADP-bound structures on the allosteric domain (left) show minimal differences at the protomer level. f. Comparison of phosphate- (dark grey) and ADP-bound (orange/blue) structures to human crystal structures of wild type PRPS1 (light grey, PDB ID 2H06, 2HCR, 3EFH, and 3S5J). Structures have been aligned on the allosteric domain of protomer a. g. Differences in the filaments arise from the orientations of the protomers relative to each other in the hexamers, with rotations of neighboring protomers relative to a as indicated. h. Overlay of the phosphate- (dark grey) and ADP-bound (orange/blue) filament interfaces with the E. coli PRPS filament interfaces (light grey, PDB ID 7XMU, 7XMV) i. Comparison of phosphate- (dark grey) and ADP-bound (orange/blue) structures to E. coli PRPS filament structures (light grey, PDB ID 7XMV). Structures have been aligned on the allosteric domain of protomer a, and phosphates from the phosphate-bound human and E. coli structures have been omitted for clarity.
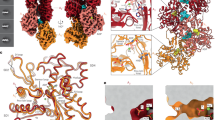
Extended Data Fig. 4 Substrate- and product-bound filaments.
a. Volume of PRPS1 filaments bound to phosphate/ATP (left), phosphate/ATP/R5P (middle), or phosphate/PRPP (right); protomers colored in blue or orange. b, top. Volume of one hexamer from a filament of PRPS1 bound to PRPP. Protomers are orange and blue, with the active site in yellow. b, bottom. Zoom in of active site indicated in (top), including the catalytic loop (dark blue), ATP (yellow), phosphate, magnesium, and coordinated waters. c–e. Volume showing the catalytic loop (dark blue or dark orange) and the ligands in the active site (yellow) for each of the filament structures presented in this work. f. Overlay of active sites shown in Main Text Fig. 3b–d and also including PRPS1 + ADP (light grey). g. Volume (top) describing location of slices (bottom) showing catalytic domains in two maps with well-resolved catalytic loops. h. Overlay of PRPS1 + ATP/R5P closed catalytic loop and key residues from the three PRPS structures from the PDB that also contain a closed catalytic loop (3MBI from Thermoplasma volcanium; 5T3O and 7PN0 from Thermus thermophilus). PRPS1 with ATP/R5P in blue, PDB models in grey. i. Overlay of PRPS1 with ATP/R5P (blue/orange) and 5T3O and 7PN0 from Thermus thermophilus (greys), showing the neighboring open and closed catalytic loops.
Extended Data Fig. 5 Example classification scheme and directional FSCs.
a. Classification scheme for PRPS1 + ATP/R5P after symmetry expansion. Particles were classified into ten classes, without alignment using a protomer mask. a subset of the resulting volumes was locally refined using a hexamer mask and exported to Phenix for density modification. Two volumes were then used for model building. b. Directional FSC for volumes derived from tilted datasets and volumes and models from the active sites from protomer a of each map.
Extended Data Fig. 6 Ligand volumes from substrate- and product-bound filaments.
Panels show the volume and the ligands for (a) PRPS1 + ATP, (b) PRPS1 + ATP/R5P with open loop, (c) PRPS1 + ATP/R5P with closed loop, (d) PRPS1 + AMP/PRPP with closed loop, (e) PRPS1 + PRPP.
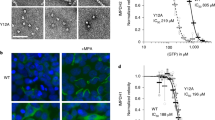
Extended Data Fig. 7 Mutation of filament interface residues.
a. Panel of negative stain EM sections of PRPS1 engineered mutations in phosphate buffer in the presence of the indicated ligands. b. Chromatography curves from a Superose 6 of PRPS1 and three engineered, filament-interface mutations. c. Assay performed in buffer containing: 50 mM Potassium HEPES pH 7.6, 6 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 0.1 mg/mL bovine serum albumin. Left: Activity assay of the three engineered mutations with or without 50 mM potassium phosphate, pH 7.6 (N = 4 technical replicates). Right: Ratio of 50 mM phosphate: 0 mM phosphate activities (V) from the panel to the left. d. Substrate kinetics of the three engineered mutations at protein concentrations with detectable catalytic activity. Assay performed in buffer containing: 50 mM Potassium Phosphate pH 7.6, 6 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 0.1 mg/mL bovine serum albumin. Triplicate readings of one well for a single replicate (N = 1 technical replicate) are shown as open circles. e. Kinetic parameters for the wild type protein and the three engineered mutations.
Extended Data Fig. 8 Control assays for catalysis experiments.
a. PRPS1 catalysis over time at the lowest ribose-5-phosphate concentration used (100 μM ATP, 1.5 μM ribose-5-phosphate), plotted before conversion to μM AMP. Individual data points are shown as open circles (N = 3). b. PRPS1 kinetic analysis varying ATP concentration and holding ribose-5-phosphate at 100 μM. Individual data points are shown as open circles. Solid circles and error bars represent mean ± standard deviation (N = 3). Calculated kinetic parameters in inset.
Extended Data Fig. 9 Volumes for C-termini of PRPS1-E307A mutations.
a–d. Panels detailing PRPS1-E307A maps and models, with protomers in blue/orange and C-termini highlighted in red. Row 1: Dataset, symmetry, and number of particles included in the map. Row 2: View of one face of the ResMap filtered volumes from PRPS1-E307A datasets. Row 3: Insert showing volume of C-termini of protomer a from Relion’s implementation of ResMap (grey box) or Phenix’s Density Modification (black/white box). Rows 4 & 5: View of both faces of the density modified volumes from PRPS1-E307A datasets.
Extended Data Fig. 10 Filament formation in PRPS1 disease mutants.
a. Kinetic parameters for the wild type protein and the four disease mutations as determined from the data shown in Main Text Fig. 6. b. Chromatography curves from a Superose 6 of PRPS1 and four disease mutations. c. Panel of negative stain EM sections of PRPS1 disease mutations in phosphate buffer in the presence of the indicated ligands.
Supplementary information
Supplementary Video 1
Morph between phosphate-bound PRPS1 filament and ADP-bound PRPS1 filament. Cartoon of backbone atoms, with the protomers colored in orange and blue, and a phosphate positioned in the allosteric site for reference. Filaments have been aligned at the central interface. The video progresses as follows: phosphate-bound to ADP-bound to phosphate-bound to ADP-bound.
Supplementary Video 2
Morph between the open and closed conformations of PRPS1 incubated with ATP and ribose-5-phosphate. Top and side views of cartoon of backbone atoms of hexamer, with the protomers colored in orange and blue and the catalytic loops in the a and b protomers in dark orange and dark blue, respectively. Active site ligands are shown in yellow. Hexamers have been aligned on protomers c–f. The video progresses from protomer b in the open-loop conformation to protomer b in the closed-loop conformation, back to protomer b in the open-loop conformation to protomer b in the closed-loop conformation.
Supplementary Table 1
Primers used in this manuscript.
Source data
Source Data Fig. 4
Activity assay data points.
Source Data Fig. 6
Activity assay data points.
Source Data Extended Data Fig. 1
Size exclusion chromatography curve data.
Source Data Extended Data Fig. 2
Fourier shell correlation curve data points.
Source Data Extended Data Fig. 5
Three-dimensional Fourier shell correlation curve data points and histogram data.
Source Data Extended Data Fig. 7
Activity assay data points and size exclusion chromatography curve data.
Source Data Extended Data Fig. 8
Activity assay data points.
Source Data Extended Data Fig. 10
Size exclusion chromatography curve data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hvorecny, K.L., Hargett, K., Quispe, J.D. et al. Human PRPS1 filaments stabilize allosteric sites to regulate activity. Nat Struct Mol Biol 30, 391–402 (2023). https://doi.org/10.1038/s41594-023-00921-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-00921-z
This article is cited by
-
A three-level regulatory mechanism of the aldo-keto reductase subfamily AKR12D
Nature Communications (2024)
-
Filament formation drives catalysis by glutaminase enzymes important in cancer progression
Nature Communications (2024)
-
Direct stimulation of de novo nucleotide synthesis by O-GlcNAcylation
Nature Chemical Biology (2024)
-
Structural basis of human PRPS2 filaments
Cell & Bioscience (2023)