Abstract
Binding of the neurotransmitter acetylcholine to its receptors on muscle fibers depolarizes the membrane and thereby triggers muscle contraction. We sought to understand at the level of three-dimensional structure how agonists and antagonists alter nicotinic acetylcholine receptor conformation. We used the muscle-type receptor from the Torpedo ray to first define the structure of the receptor in a resting, activatable state. We then determined the receptor structure bound to the agonist carbachol, which stabilizes an asymmetric, closed channel desensitized state. We find conformational changes in a peripheral membrane helix are tied to recovery from desensitization. To probe mechanisms of antagonism, we obtained receptor structures with the active component of curare, a poison arrow toxin and precursor to modern muscle relaxants. d-Tubocurarine stabilizes the receptor in a desensitized-like state in the presence and absence of agonist. These findings define the transitions between resting and desensitized states and reveal divergent means by which antagonists block channel activity of the muscle-type nicotinic receptor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Cryo-EM maps and atomic model coordinates have been deposited in the Electron Microscopy Data Bank and PDB, respectively; apo (EMD-25202 and PDB 7SMM), apo plus cholesterol (EMD-25205 and PDB 7SMQ), carbachol-bound desensitized state (EMD-25206 and PDB 7SMR), d-tubo bound (EMD-25207 and PDB 7SMS) and d-tubo plus carbachol bound (EMD-25208 and PDB 7SMT). Source data are provided with this paper.
References
Gharpure, A., Noviello, C. M. & Hibbs, R. E. Progress in nicotinic receptor structural biology. Neuropharmacology 171, 108086 (2020).
Albuquerque, E. X., Pereira, E. F., Alkondon, M. & Rogers, S. W. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 89, 73–120 (2009).
Karlin, A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3, 102–114 (2002).
Katz, B. & Thesleff, S. A study of the desensitization produced by acetylcholine at the motor end-plate. J. Physiol. 138, 63–80 (1957).
Raghavendra, T. Neuromuscular blocking drugs: discovery and development. J. R. Soc. Med. 95, 363–367 (2002).
Bennett, M. R. The concept of transmitter receptors: 100 years on. Neuropharmacology 39, 523–546 (2000).
King, H. Curare. Nature 135, 469–470 (1935).
King, H. 330. Curare alkaloids. Part I. Tubocurarine. J. Chem. Soc. https://doi.org/10.1039/JR9350001381 (1935).
Rahman, M. M. et al. Structure of the native muscle-type nicotinic receptor and inhibition by snake venom toxins. Neuron 106, 952–962 e955 (2020).
Rahman, M. M., Worrell, B. T., Stowell, M. H. B. & Hibbs, R. E. in Methods in Enzymology 653 (eds Minor, D. L. & Colecraft, H. M.) 189–206 (Academic Press, 2021).
Raftery, M. A., Hunkapiller, M. W., Strader, C. D. & Hood, L. E. Acetylcholine receptor: complex of homologous subunits. Science 208, 1454–1456 (1980).
Noda, M. et al. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature 302, 528–532 (1983).
Miyazawa, A., Fujiyoshi, Y., Stowell, M. & Unwin, N. Nicotinic acetylcholine receptor at 4.6 A resolution: transverse tunnels in the channel wall. J. Mol. Biol. 288, 765–786 (1999).
Changeux, J. P. The nicotinic acetylcholine receptor: a typical ‘allosteric machine’. Philos. Trans. R. Soc. Lond. B. Biol. Sci. https://doi.org/10.1098/rstb.2017.0174 (2018).
Brannigan, G., Henin, J., Law, R., Eckenhoff, R. & Klein, M. L. Embedded cholesterol in the nicotinic acetylcholine receptor. Proc. Natl Acad. Sci. USA 105, 14418–14423 (2008).
Baenziger, J. E., Domville, J. A. & Therien, J. P. D. The role of cholesterol in the activation of nicotinic acetylcholine receptors. Curr. Top. Membr. 80, 95–137 (2017).
Hamouda, A. K., Sanghvi, M., Sauls, D., Machu, T. K. & Blanton, M. P. Assessing the lipid requirements of the Torpedo californica nicotinic acetylcholine receptor. Biochemistry 45, 4327–4337 (2006).
Unwin, N. Protein-lipid architecture of a cholinergic postsynaptic membrane. IUCrJ 7, 852–859 (2020).
Sridhar, A. et al. Regulation of a pentameric ligand-gated ion channel by a semi-conserved cationic-lipid binding site. J. Biol. Chem. https://doi.org/10.1016/j.jbc.2021.100899 (2021).
Zhong, W. et al. From ab initio quantum mechanics to molecular neurobiology: a cation-pi binding site in the nicotinic receptor. Proc. Natl Acad. Sci. USA 95, 12088–12093 (1998).
Howard, R. J. Elephants in the Dark: Insights and incongruities in pentameric ligand-gated ion channel models. J. Mol. Biol. https://doi.org/10.1016/j.jmb.2021.167128 (2021).
Nemecz, A., Prevost, M. S., Menny, A. & Corringer, P. J. Emerging molecular mechanisms of signal transduction in pentameric ligand-gated ion channels. Neuron 90, 452–470 (2016).
Mitra, A., Cymes, G. D. & Auerbach, A. Dynamics of the acetylcholine receptor pore at the gating transition state. Proc. Natl Acad. Sci. USA 102, 15069–15074 (2005).
Grandl, J., Danelon, C., Hovius, R. & Vogel, H. Functional asymmetry of transmembrane segments in nicotinic acetylcholine receptors. Eur. Biophys. J. 35, 685–693 (2006).
Grosman, C. & Auerbach, A. Asymmetric and independent contribution of the second transmembrane segment 12’ residues to diliganded gating of acetylcholine receptor channels: a single-channel study with choline as the agonist. J. Gen. Physiol. 115, 637–651 (2000).
Unwin, N., Miyazawa, A., Li, J. & Fujiyoshi, Y. Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the alpha subunits. J. Mol. Biol. 319, 1165–1176 (2002).
Unwin, N. & Fujiyoshi, Y. Gating movement of acetylcholine receptor caught by plunge-freezing. J. Mol. Biol. 422, 617–634 (2012).
Noviello, C. M. et al. Structure and gating mechanism of the alpha7 nicotinic acetylcholine receptor. Cell 184, 2121–2134 e2113 (2021).
Dwyer, T. M., Adams, D. J. & Hille, B. The permeability of the endplate channel to organic cations in frog muscle. J. Gen. Physiol. 75, 469–492 (1980).
Revah, F. et al. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature 353, 846–849 (1991).
Yakel, J. L., Lagrutta, A., Adelman, J. P. & North, R. A. Single amino acid substitution affects desensitization of the 5-hydroxytryptamine type 3 receptor expressed in Xenopus oocytes. Proc. Natl Acad. Sci. USA 90, 5030–5033 (1993).
Basak, S. et al. Cryo-EM structure of 5-HT3A receptor in its resting conformation. Nat. Commun. 9, 514 (2018).
Wilson, G. & Karlin, A. Acetylcholine receptor channel structure in the resting, open, and desensitized states probed with the substituted-cysteine-accessibility method. Proc. Natl Acad. Sci. USA 98, 1241–1248 (2001).
Zhao, Y. et al. Structural basis of human alpha7 nicotinic acetylcholine receptor activation. Cell Res. 31, 713–716 (2021).
Kumar, A. et al. Mechanisms of activation and desensitization of full-length glycine receptor in lipid nanodiscs. Nat. Commun. 11, 3752 (2020).
Auerbach, A. & Akk, G. Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gate mechanism. J. Gen. Physiol. 112, 181–197 (1998).
Gharpure, A. et al. Agonist selectivity and ion permeation in the alpha3beta4 ganglionic nicotinic receptor. Neuron, https://doi.org/10.1016/j.neuron.2019.07.030 (2019).
Morales-Perez, C. L., Noviello, C. M. & Hibbs, R. E. X-ray structure of the human alpha4beta2 nicotinic receptor. Nature 538, 411–415 (2016).
Walsh, R. M. Jr. et al. Structural principles of distinct assemblies of the human alpha4beta2 nicotinic receptor. Nature 557, 261–265 (2018).
Polovinkin, L. et al. Conformational transitions of the serotonin 5-HT3 receptor. Nature 563, 275–279 (2018).
Basak, S., Gicheru, Y., Rao, S., Sansom, M. S. P. & Chakrapani, S. Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT3A receptor. Nature 563, 270–274 (2018).
Kim, J. J. et al. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 585, 303–308 (2020).
Masiulis, S. et al. GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 565, 454–459 (2019).
Thompson, M. J., Domville, J. A. & Baenziger, J. E. The functional role of the alphaM4 transmembrane helix in the muscle nicotinic acetylcholine receptor probed through mutagenesis and coevolutionary analyses. J. Biol. Chem. 295, 11056–11067 (2020).
Carswell, C. L. et al. Role of the fourth transmembrane alpha helix in the allosteric modulation of pentameric ligand-gated ion channels. Structure 23, 1655–1664 (2015).
Butler, A. S. et al. Importance of the C-terminus of the human 5-HT3A receptor subunit. Neuropharmacology 56, 292–302 (2009).
Cory-Wright, J. et al. Aromatic residues in the fourth transmembrane-spanning helix M4 Are important for GABArho receptor function. ACS Chem. Neurosci. 9, 284–290 (2018).
Pons, S. et al. Critical role of the C-terminal segment in the maturation and export to the cell surface of the homopentameric alpha 7-5HT3A receptor. Eur. J. Neurosci. 20, 2022–2030 (2004).
Jenkinson, D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J. Physiol. 152, 309–324 (1960).
Pedersen, S. E. & Papineni, R. V. Interaction of d-tubocurarine analogs with the Torpedo nicotinic acetylcholine receptor. Methylation and stereoisomerization affect site-selective competitive binding and binding to the noncompetitive site. J. Biol. Chem. 270, 31141–31150 (1995).
Moore, M. A. & McCarthy, M. P. Snake venom toxins, unlike smaller antagonists, appear to stabilize a resting state conformation of the nicotinic acetylcholine receptor. Biochim. Biophys. Acta 1235, 336–342 (1995).
Sine, S. M. & Steinbach, J. H. Acetylcholine receptor activation by a site-selective ligand: nature of brief open and closed states in BC3H-1 cells. J. Physiol. 370, 357–379 (1986).
Steinbach, J. H. & Chen, Q. Antagonist and partial agonist actions of d-tubocurarine at mammalian muscle acetylcholine receptors. J. Neurosci. 15, 230–240 (1995).
Neubig, R. R. & Cohen, J. B. Equilibrium binding of [3H]tubocurarine and [3H]acetylcholine by Torpedo postsynaptic membranes: stoichiometry and ligand interactions. Biochemistry 18, 5464–5475 (1979).
Chiara, D. C. & Cohen, J. B. Identification of amino acids contributing to high and low affinity d-tubocurarine sites in the Torpedo nicotinic acetylcholine receptor. J. Biol. Chem. 272, 32940–32950 (1997).
Sine, S. M. & Claudio, T. Gamma- and delta-subunits regulate the affinity and the cooperativity of ligand binding to the acetylcholine receptor. J. Biol. Chem. 266, 19369–19377 (1991).
Chiara, D. C., Xie, Y. & Cohen, J. B. Structure of the agonist-binding sites of the Torpedo nicotinic acetylcholine receptor: affinity-labeling and mutational analyses identify gamma Tyr-111/delta Arg-113 as antagonist affinity determinants. Biochemistry 38, 6689–6698 (1999).
Sine, S. M. Molecular dissection of subunit interfaces in the acetylcholine receptor: identification of residues that determine curare selectivity. Proc. Natl Acad. Sci. USA 90, 9436–9440 (1993).
Strecker, G. J. & Jackson, M. B. Curare binding and the curare-induced subconductance state of the acetylcholine receptor channel. Biophys. J. 56, 795–806 (1989).
Colquhoun, D., Dreyer, F. & Sheridan, R. E. The actions of tubocurarine at the frog neuromuscular junction. J. Physiol. 293, 247–284 (1979).
Sine, S. M. & Taylor, P. Relationship between reversible antagonist occupancy and the functional capacity of the acetylcholine receptor. J. Biol. Chem. 256, 6692–6699 (1981).
Gielen, M., Barilone, N. & Corringer, P. J. The desensitization pathway of GABAA receptors, one subunit at a time. Nat. Commun. 11, 5369 (2020).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife https://doi.org/10.7554/eLife.42166 (2018).
Frauenfeld, J. et al. A saposin-lipoprotein nanoparticle system for membrane proteins. Nat. Methods 13, 345–351 (2016).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. GCTF: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Celie, P. H. et al. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41, 907–914 (2004).
Brams, M. et al. A structural and mutagenic blueprint for molecular recognition of strychnine and d-tubocurarine by different cys-loop receptors. PLoS Biol. 9, e1001034 (2011).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol. 74, 531–544 (2018).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph 14, 354–360 (1996). 376.
Wallace, A. C., Laskowski, R. A. & Thornton, J. M. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 8, 127–134 (1995).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Gao, F. et al. Curariform antagonists bind in different orientations to acetylcholine-binding protein. J. Biol. Chem. 278, 23020–23026 (2003).
Basta, T. et al. Self-assembled lipid and membrane protein polyhedral nanoparticles. Proc. Natl Acad. Sci. USA 111, 670–674 (2014).
McWilliam, H. et al. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 41, W597–W600 (2013).
Crooks, G. E., Hon, G., Chandonia, J. M. & Brenner, S. E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Acknowledgements
We thank S. Sine for Torpedo receptor cDNAs, and C. Noviello and S. Zhu for assistance in cryo-EM sample screening. We are grateful to S. Burke, J.J. Kim, C. Noviello, S. Sine and M. Klymkowsky for critical feedback on the manuscript, D. Borek for model building discussion and A. Sobolevsky for helpful discussion related to measuring recovery from desensitization. Single-particle cryo-EM grids were screened at the University of Texas Southwestern Medical Center Cryo-Electron Microscopy Facility, which is supported by the CPRIT Core Facility Support award no. RP170644. We thank H. Scott for cryo-EM data collection at the PNCC under user proposal nos. 50839 and 51574. A portion of this research was supported by National Institutes of Health (NIH) grant no. U24GM129547 and performed at the PNCC at the Oregon Health & Science University and accessed through EMSL (grid.436923.9), a Department of Energy Office of Science User Facility sponsored by the Office of Biological and Environmental Research. M.M.R. acknowledges a postdoctoral fellowship from the American Heart Association (no. 827474). This work was supported by grants from the NIH (nos. DA042072 to R.E.H., AG061829 to M.H.B.S. and NS120496 to R.E.H. and M.H.B.S.) and the MCDB Neurodegenerative Disease Fund to M.H.B.S.
Author information
Authors and Affiliations
Contributions
M.M.R. performed the sample preparation and data processing for cryo-EM, structural analysis and drafted the manuscript with R.E.H. J.T. performed the electrophysiology. B.T.W. synthesized the ATM affinity reagent. T.B. and M.L. performed the lipid quantification. M.H.B.S. and R.E.H. assisted in structural analysis and model validation and directed the project. M.M.R., R.E.H. and M.H.B.S. revised the manuscript with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural and Molecular Biology thanks Michaela Jansen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Florian Ullrich was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Relion 3.1 workflow for data processing.
Representative processing approach for apo dataset.
Extended Data Fig. 2 Local and global resolution estimates for cryo-EM maps.
Local resolution is illustrated by variation in map surface color and was estimated using RELION. Global resolution was estimated at FSC = 0.143 (dotted line) from half maps.
Extended Data Fig. 3 Lipid-receptor interactions.
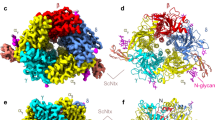
a, Cholesterol (red) and phospholipid (yellow) binding sites in the receptor. α subunit - green, β subunit - khaki, γ subunit - blue, δ subunit – violet. High-affinity cholesterol binding sites are near the MX helices and low-affinity binding sites are near outer membrane leaflet. Among the five subunit interfaces, the high affinity cholesterols occupy the same positions in three interfaces: α/γ, α/δ, and β/α, but not γ/αδ and δ/β. The environment of that site is more polar in γ and δ subunits, where an asparagine replaces I291/α or L297/β (in M3). b, Conservation of the residues in the cholesterol high affinity binding site, residues numbers are according to T. californica α-subunit. Accession numbers for sequences used to determine conservation are given in the Methods.
Extended Data Fig. 4 Transition between resting and desensitized states.
a, Two electrode voltage clamp (TEVC) recording show activation and desensitization of the Torpedo nicotinic receptor by carbachol; 5 mM carbachol was supplemented into the cryo-EM sample for structure determination of the desensitized state. b, Asymmetry in the TMD conformational change between resting and desensitized states; residues at the 16′ position are shown as sticks. c, Conformational changes in loop C of an α-subunit and Loop F of a complementary subunit after agonist and antagonist binding compared to resting state. Carbachol is shown as green spheres. d, Conformational differences in a representative coupling region; representative α and γ subunits.
Extended Data Fig. 5 Permeation pathway and pore profile.
a, Pore diameter comparison of Torpedo in resting and desensitized states vs. other Cys-loop receptors: resting, α7 nAChR (PDB ID: 7EKI), 5-HT3AR (PDB ID: 6BE1), GlyR (PDB ID: 6UBS); and desensitized, α7 nAChR (PDB ID: 7KOQ), α4β2 nAChR (PDB ID: 6CNJ), α3β4 nAChR (PDB ID: 6PV7), 5-HT3AR (PDB ID: 6HIQ), GlyR (PDB ID: 6UBT). b, Permeation pathway cutaway colored by electrostatic potential. c, Water molecules at the 2′ gate of the desensitized state structure. d, −1′ residue orientation in the Torpedo receptor in resting and desensitized states. Transparent surface is experimental density map. e, Two α-subunits superposition of resting state structure. f, Dose response parameters measured by TEVC of WT and mutants. Nonlinear regression was carried out using GraphPad Prism 8. Replicate measurements are from independent oocytes.
Extended Data Fig. 6 Receptor activation and antagonism by carbachol and d-tubo; d-tubo binding sites with corresponding density maps.
a, Two-electrode voltage clamp (TEVC) recording illustrates receptor antagonism by d-tubo. b, d-Tubo at α/γ interface (site 1). c, d-Tubo at α/δ interface (site 2). d, d-Tubo in the pore (site 3). e, d-Tubo at junction of M1, M3 and M4 helices of the αγ subunit (site 4). Corresponding d-tubo densities are shown as semitransparent surfaces. The d-tubo density was very clear at sites 1 and 4. Site 2 density suggested two different orientations of d-tubo and we modeled the best fitted one. The density at site 3 is poorly resolved suggesting multiple orientations of d-tubo in the pore; we used the unsharpened map to model d-tubo there. Density map was contoured at a threshold of 0.02 for site 1, 2 & 4 and 0.013 for site 3 in UCSF chimera.
Extended Data Fig. 7 Orthosteric binding-sites details and carbachol vs. d-tubo complex superposition.
a, b, Electrostatic potential of the two orthosteric ligand-binding sites in the apo form; binding pockets are indicated by asterisks. c, Residue differences between two orthosteric ligand-binding sites; αγ/γ as colored (α- green, γ- blue) and αδ/δ as gray. d, Superposition of desensitized and d-tubo bound structures; d-Tubo model is colored (α, green; β, khaki; γ, blue; δ, violet) and desensitized structure is in gray. (e) Conformational difference in M4 of two α-subunits in resting, desensitized and pure d-tubo bound structures.
Supplementary information
Supplementary Video 1
Structural transition between resting and desensitized states. Morphing video illustrates changes from resting state to desensitized state to resting state in the top view. α subunit, green; β subunit, khaki; γ subunit, blue and δ subunit, violet.
Supplementary Video 2
Structural transition between resting and desensitized states. Morphing video illustrates changes from resting state to desensitized state to resting state in the side view. α subunit, green; β subunit, khaki; γ subunit, blue and δ subunit, violet.
Supplementary Video 3
d-Tubo interactions at the α–γ interface (site 1). d-Tubo is shown as sticks (orange) and corresponding densities are shown as semitransparent surfaces. Interacting residues are also shown as sticks and colored by subunits: α subunit, green and γ subunit, blue.
Supplementary Video 4
d-Tubo interactions at the α–δ interface (site 2). d-Tubo is shown as sticks (orange) and corresponding densities are shown as semitransparent surfaces. Interacting residues are also shown as sticks and colored by subunits: α subunit, green and δ subunit, violet.
Supplementary Video 5
d-Tubo interactions at the pore (site 3). d-Tubo is shown as sticks (orange) and corresponding densities are shown as semitransparent surfaces. Interacting residues are also shown as sticks and colored by subunits: α subunit, green; β subunit, khaki; γ subunit, blue and δ subunit, violet.
Supplementary Video 6
d-Tubo interactions at the M1, M3 and M4 helices of αγ subunit (site 4). d-Tubo is shown as sticks (orange) and corresponding densities are shown as semitransparent surfaces. Interacting residues are also shown as sticks and colored by subunit: α subunit, green.
Source data
Source Data Fig. 1
Cholesterol and phospholipid assay.
Source Data Fig. 4
Dose–response curve and desensitization recovery.
Rights and permissions
About this article
Cite this article
Rahman, M.M., Basta, T., Teng, J. et al. Structural mechanism of muscle nicotinic receptor desensitization and block by curare. Nat Struct Mol Biol 29, 386–394 (2022). https://doi.org/10.1038/s41594-022-00737-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-022-00737-3
This article is cited by
-
State-dependent binding of cholesterol and an anionic lipid to the muscle-type Torpedo nicotinic acetylcholine receptor
Communications Biology (2024)
-
Cryo-EM structures of prokaryotic ligand-gated ion channel GLIC provide insights into gating in a lipid environment
Nature Communications (2024)
-
A release of local subunit conformational heterogeneity underlies gating in a muscle nicotinic acetylcholine receptor
Nature Communications (2024)
-
Lipid nanodisc scaffold and size alter the structure of a pentameric ligand-gated ion channel
Nature Communications (2024)
-
The modes of action of ion-channel-targeting neurotoxic insecticides: lessons from structural biology
Nature Structural & Molecular Biology (2023)