Abstract
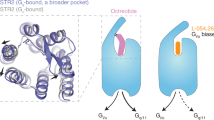
Somatostatin is a signaling peptide that plays a pivotal role in physiologic processes relating to metabolism and growth through its actions at somatostatin receptors (SSTRs). Members of the SSTR subfamily, particularly SSTR2, are key drug targets for neuroendocrine neoplasms, with synthetic peptide agonists currently in clinical use. Here, we show the cryogenic-electron microscopy structures of active-state SSTR2 in complex with heterotrimeric Gi3 and either the endogenous ligand SST14 or the FDA-approved drug octreotide. Complemented by biochemical assays and molecular dynamics simulations, these structures reveal key details of ligand recognition and receptor activation at SSTRs. We find that SSTR ligand recognition is highly diverse, as demonstrated by ligand-induced conformational changes in ECL2 and substantial sequence divergence across subtypes in extracellular regions. Despite this complexity, we rationalize several known sources of SSTR subtype selectivity and identify an additional interaction for specific binding. These results provide valuable insights for structure-based drug discovery at SSTRs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data for all assays has been provided in spreadsheet format and raw micrographs have been uploaded to EMPIAR under the accession codes EMPIAR-10931 and EMPIAR-10932. All data generated or analyzed in this study are included in this article and the Supplementary Information. The cryo-EM density maps and corresponding coordinates have been deposited in the Electron Microscopy Data Bank (EMDB) and the PDB, respectively, under the following accession codes: PDB 7T10 EMDB-25586 (SST14) and PDB 7T11 EMDB-25587 (Octreotide). Source data are provided with this paper.
Change history
08 July 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41594-022-00813-8
References
Günther, T. et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin receptors: structure, function, ligands, and new nomenclature. Pharmacol. Rev. 70, 762–835 (2018).
Gu, Y. Z. & Schonbrunn, A. Coupling specificity between somatostatin receptor sst2A and G proteins: Isolation of the receptor-G protein complex with a receptor antibody. Mol. Endocrinol. 11, 527–537 (1997).
Hofman, M. S., Eddie Lau, W. F. & Hicks, R. J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 35, 500–516 (2015).
Kaltsas, G., Androulakis, I. I., De Herder, W. W. & Grossman, A. B. Paraneoplastic syndromes secondary to neuroendocrine tumours. Endocr. Relat. Cancer 17, 173–193 (2010).
Liapakis, G. et al. Identification of ligand binding determinants in the somatostatin receptor subtypes 1 and 2. J. Biol. Chem. 271, 20331–20339 (1996).
Bruns, C., Lewis, I., Briner, U., Meno-Tetang, G. & Weckbecker, G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 146, 707–716 (2002).
Casarini, A. P. M. et al. Acromegaly: correlation between expression of somatostatin receptor subtypes and response to octreotide-lar treatment. Pituitary 12, 297–303 (2009).
Plöckinger, U., Dienemann, D. & Quabbe, H.-J. Gastrointestinal side-effects of octreotide during long term treatment of acromegaly. J. Clin. Endocrinol. Metab. 71, 1658–1662 (1990).
Parry, J. J., Chen, R., Andrews, R., Lears, K. A. & Rogers, B. E. Identification of critical residues involved in ligand binding and G protein signaling in human somatostatin receptor subtype 2. Endocrinol. 153, 2747–2755 (2012).
Strnad, J. & Hadcock, J. R. Identification of a critical aspartate residue in transmembrane domain three necessary for the binding of somatostatin to the somatostatin receptor SSTR2. Biochem. Biophys. Res. Commun. 22, 913–921 (1995).
Greenwood, M. T. et al. Ligand binding pocket of the human somatostatin receptor 5: Mutational analysis of the extracellular domains. Mol. Pharmacol. 52, 807–814 (1997).
Nehrung, R. B., Meyerhof, W. & Richter, D. Aspartic acid residue 124 in the third transmembrane domain of the somatostatin receptor subtype 3 is essential for somatostatin-14 binding. DNA Cell Biol. 14, 939–944 (1995).
Kaupmann, K. et al. Two amino acids, located in transmembrane domains VI and VII, determine the selectivity of the peptide agonist SMS 201-995 for the SSTR2 somatostatin receptor. EMBO J. 14, 727–735 (1995).
Olsen, R. H. J. et al. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 16, 841–849 (2020).
Che, T. et al. Nanobody-enabled monitoring of kappa opioid receptor states. Nat. Commun. 11, 1145 (2020).
Robertson, M. J. et al. Structure determination of inactive-state GPCRs with a universal nanobody. Preprint at bioRxiv https://doi.org/10.1101/2021.11.02.466983 (2021).
Maeda, S. et al. Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat. Commun. 9, 3712 (2018).
Maeda, S., Qu, Q., Robertson, M. J., Skiniotis, G. & Kobilka, B. K. Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 364, 552–557 (2019).
Koehl, A. et al. Structure of the μ-opioid receptor-Gi protein complex. Nature 558, 547–552 (2018).
Ballesteros, J. A. & Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 (1995).
Michel, J., Tirado-Rives, J. & Jorgensen, W. L. Prediction of the water content in protein binding sites. J. Phys. Chem. B. 113, 13337–13346 (2009).
Wacker, D. et al. Structural features for functional selectivity at serotonin receptors. Science 340, 615–619 (2013).
Deupi, X., Standfuss, J. & Schertler, G. Conserved activation pathways in G-protein-coupled receptors. Biochem. Soc. Trans. 40, 383–388 (2012).
McAllister, S. D. et al. Structural mimicry in class A G protein-coupled receptor rotamer toggle switches. J. Biol. Chem. 46, 48024–48037 (2004).
Xing, C. et al. Cryo-EM structure of the human cannabinoid receptor CB2-Gi signaling complex. Cell 180, 645–654 (2020).
Xu, X. et al. Binding pathway determines norepinephrine selectivity for the human β1AR over β2AR. Cell Res. 31, 569–579 (2021).
Zivanov, J. et al. RELION-3: new tools for automated high-resolution cryo-EM structure determination. eLife. 7, e42166 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: agorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Emsley, P. & Cowtan, K. Coot: model=building tools for molecular graphics. Acta Crystallogr. D. Struct. Biol. 60, 2126–2132 (2004).
Liebshner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D. Struct. Biol. 75, 861–877 (2019).
Robertson, M. J., van Zundert, G. C. P., Borrelli, K. & Skiniotis, G. GemSpot: a pipeline for robust modeling of ligands into Cryo-EM Maps. Structure 28, 707–716 (2020).
Sindhikara, D. et al. Improving accuracy, diversity, and speed with prime macrocycle conformational sampling. J. Chem. Inf. Model. 57, 1881–1894 (2017).
van Zundert, G. C. P., Moriarty, N. W., Sobolev, O. V., Adams, P. D. & Borrelli, K. W. Macromolecular refinement of X-ray and cryoelectron microscopy structures with Phenix/OPLS3e for improved structure and ligand quality. Structure 29, 913–921 (2021).
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I. & Lomize, A. L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. https://doi.org/10.1093/nar/gkr703 (2012).
Lee, J. et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 12, 405–413 (2016).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2016).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Miyamoto, S. & Kollman, P. A. Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 13, 952–962 (1992).
Ryckaert, J. P., Ciccotti, G. & Berendsen, H. J. C. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Robertson, M. J., Tirado-Rives, J. & Jorgensen, W. L. Improved peptide and protein torsional energetics with the OPLS-AA force field. J. Chem. Theory Comput. 11, 3499–3509 (2015).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Jorgensen, W. L. & Tirado-Rives, J. Molecular modeling of organic and biomolecular systems using BOSS and MCPRO. J. Comput. Chem. 26, 1689–1700 (2005).
Towns, J. et al. XSEDE: accelerating scientific discovery. Comput. Sci. Eng. 16, 62–74 (2014).
Acknowledgements
Cryo-EM data were collected at the Stanford cryo-EM center (cEMc) with support from E. Montabana. This work was supported, in part, by the Mathers Foundation (G.S.), training grant no. T32GM089626 (J.G.M.) and used the Extreme Science and Engineering Discovery Environment (XSEDE)46 resource comet-gpu through sdsc-comet allocation grant no. TG-MCB190153 (G.S.), which is supported by National Science Foundation grant number ACI-1548562.
Author information
Authors and Affiliations
Contributions
M.J.R. cloned constructs, expressed and purified proteins, processed electron microscopy data, built models and ran then analyzed molecular dynamics simulations. J.G.M. performed BRET assays, expressed and purified proteins. O.P. prepared cryo-EM samples and collected cryo-EM data. K.B., M.J.R. and G.S. wrote the paper with input from J.G.M. and O.P. G.S. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural and Molecular Biology thanks Daniel Rosenbaum and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Florian Ullrich was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 G protein specificity of SSTR2.
a, Dose-response curves of G protein-specific activation pathways of SSTR2 by SST14 (magenta squares), or octreotide (lavender circles), as compared to activation of neurotensin 1 receptor (NTSR1) by neurotensin (blue triangles) or 𝜷2 adrenergic receptor by isoproterenol (teal diamonds) from simultaneous curve fitting of n = 3 biologically independent replicates. Error bars are S.E.M.. b, Bar plot of Emax from the above dose-response curves. Data represent mean + /− S.E.M. from the simultaneous curve-fitting of n = 3 biological replicates.
Extended Data Fig. 2 SSTR2/Gi3/scFv16/octreotide complex cryo-EM data collection and processing.
a, Representative micrograph of SSTR2/Gi3/scFv16/octreotide complex, 1 micrograph out of 7,576. b, Example final 2D classes of SSTR2/Gi3/scFv16/octreotide complex. c, Cryo-EM data processing workflow. d, Local resolution of SSTR2/Gi3/scFv16/octreotide global refinement with FSC curve below. e, Local resolution of SSTR2/Gi3/scFv16/octreotide local refinement on SSTR2 with FSC curve below.
Extended Data Fig. 3 SSTR2/Gi3/scFv16/SST14 complex cryo-EM data collection and processing.
a, Representative micrograph of SSTR2/Gi3/scFv16/SST14 complex, 1 micrograph out of 9,740. b, Example final 2D classes of SSTR2/Gi3/scFv16/SST14 complex. c, Cryo-EM data processing workflow. d, Local resolution of SSTR2/Gi3/scFv16/SST14 global refinement with FSC curve below.
Extended Data Fig. 4 Map-Model Agreements.
a, Map-model comparison for SSTR2/SST14. b, Map-model comparison for SSTR2/Octreotide.
Extended Data Fig. 5 Comparison of SSTR2 Gi3 interface.
a, Alignment of SSTR2/Gi3 and MOR Gi1 from two different angles. b, Hydration at the SSTR2 ICL2/Gi3 interface; orange spheres are predicted water positions from JAWS simulations. c, Hydration at the SSTR2 DRY motif/Gi3 interface; orange spheres are predicted water positions from JAWS simulations.
Extended Data Fig. 6 Comparison of ECL2 and ECL3 interactions and mutagenesis with octreotide and SST14.
a, Overlay of the structures of SSTR2 bound to octreotide (lavender) and SST14 (magenta) around ECL3. b, Dose-response curves of SSTR2-dependent Gi3 BRET biosensor activation by SST14. c, Dose-response curves of SSTR2-dependent Gi3 BRET biosensor activation by octreotide. d, Cell surface expression analysis of point mutants and ECL swaps of SSTR2 and e, SSTR1. Bars represent mean value + /− S.E.M. from n = 3 biologically independent replicates. f, Dose-response curves of SSTR3-dependent Gi3 BRET biosensor activation by SST14. All dose-response curve points represent the mean + /− S.E.M. from n = 3 independent biological replicates, and curves are simultaneously fit to n = 3 independent biological replicates.
Extended Data Fig. 7 Comparison of interactions of additional SSTR2 agonists with SSTR2.
a, Fold change in selectivity in favor of SST14 versus SST28 calculated by EC50 shift of 11-point dose response curves. Bars represent mean EC50 ratio + /− 95% CI from simultaneous curve-fitting of n = 3 biologically independent replicates. b, Overlay of our homology model of lanreotide(green)-bound SSTR2 (teal) with our cryoEM structure of octreotide(lavender)-bound SSTR2. c, Interaction of F7 and the C terminus of SST14 (magenta) with Y2055.35 of SSTR2 (teal).
Supplementary information
Source data
Source Data Fig. 2
Excel file with source BRET data.
Source Data Fig. 3
Excel file with source BRET data.
Source Data Fig. 4
Excel file with source BRET data.
Source Data Extended Data Fig. 1
Excel file with source BRET data.
Source Data Extended Data Fig. 6
Excel file with source BRET data.
Source Data Extended Data Fig. 7
Excel file with source BRET data.
Rights and permissions
About this article
Cite this article
Robertson, M.J., Meyerowitz, J.G., Panova, O. et al. Plasticity in ligand recognition at somatostatin receptors. Nat Struct Mol Biol 29, 210–217 (2022). https://doi.org/10.1038/s41594-022-00727-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-022-00727-5
This article is cited by
-
Allosteric modulation and G-protein selectivity of the Ca2+-sensing receptor
Nature (2024)
-
Prospect of acromegaly therapy: molecular mechanism of clinical drugs octreotide and paltusotine
Nature Communications (2023)
-
An amide to thioamide substitution improves the permeability and bioavailability of macrocyclic peptides
Nature Communications (2023)
-
Ligand recognition mechanism of the human relaxin family peptide receptor 4 (RXFP4)
Nature Communications (2023)
-
Molecular simulations of SSTR2 dynamics and interaction with ligands
Scientific Reports (2023)