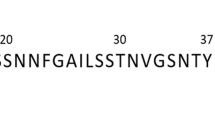Abstract
Human islet amyloid polypeptide (hIAPP) functions as a glucose-regulating hormone but deposits as amyloid fibrils in more than 90% of patients with type II diabetes (T2D). Here we report the cryo-EM structure of recombinant full-length hIAPP fibrils. The fibril is composed of two symmetrically related protofilaments with ordered residues 14–37. Our hIAPP fibril structure (i) supports the previous hypothesis that residues 20–29 constitute the core of the hIAPP amyloid; (ii) suggests a molecular mechanism for the action of the hIAPP hereditary mutation S20G; (iii) explains why the six residue substitutions in rodent IAPP prevent aggregation; and (iv) suggests regions responsible for the observed hIAPP cross-seeding with β-amyloid. Furthermore, we performed structure-based inhibitor design to generate potential hIAPP aggregation inhibitors. Four of the designed peptides delay hIAPP aggregation in vitro, providing a starting point for the development of T2D therapeutics and proof of concept that the capping strategy can be used on full-length cryo-EM fibril structures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Structural data have been deposited into the Worldwide Protein Data Bank (wwPDB) and the Electron Microscopy Data Bank (EMDB) with accession codes PDB 6VW2 and EMD-21410, respectively. Coordinates for model 2, model 1 (swap) and model 2 (swap) are available as Supplementary Data 1–3. Source data for Fig. 3b,d, Extended Data Fig. 1e,f and Extended Data Fig. 7b are available online.
Code availability
The custom software used for solvation energy calculation is available upon request.
References
Eisenberg, D. & Jucker, M. The amyloid state of proteins in human diseases. Cell 148, 1188–1203 (2012).
Roberts, A. N. et al. Molecular and functional characterization of amylin, a peptide associated with type 2 diabetes mellitus. Proc. Natl Acad. Sci. USA 86, 9662–9666 (1989).
Westermark, P. Amyloid in the islets of Langerhans: thoughts and some historical aspects. Ups. J. Med. Sci. 116, 81–89 (2011).
Westermark, P. et al. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl Acad. Sci. USA 84, 3881–3885 (1987).
Cooper, G. J. et al. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc. Natl Acad. Sci. USA 85, 7763–7766 (1988).
Höppener, J. W., Ahrén, B. & Lips, C. J. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 343, 411–419 (2000).
Maloy, A. L., Longnecker, D. S. & Greenberg, E. R. The relation of islet amyloid to the clinical type of diabetes. Hum. Pathol. 12, 917–922 (1981).
Esapa, C. et al. Islet amyloid polypeptide gene promoter polymorphisms are not associated with type 2 diabetes or with the severity of islet amyloidosis. Biochim. Biophys. Acta 1740, 74–78 (2005).
Jurgens, C. A. et al. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am. J. Pathol. 178, 2632–2640 (2011).
Westermark, P., Engström, U., Johnson, K. H., Westermark, G. T. & Betsholtz, C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc. Natl Acad. Sci. USA 87, 5036–5040 (1990).
Betsholtz, C. et al. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 251, 261–264 (1989).
Verchere, C. B. et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc. Natl Acad. Sci. USA 93, 3492–3496 (1996).
Westermark, G. T., Gebre-Medhin, S., Steiner, D. F. & Westermark, P. Islet amyloid development in a mouse strain lacking endogenous islet amyloid polypeptide (IAPP) but expressing human IAPP. Mol. Med. Camb. Mass 6, 998–1007 (2000).
Lee, S. C. et al. The islet amyloid polypeptide (amylin) gene S20G mutation in Chinese subjects: evidence for associations with type 2 diabetes and cholesterol levels. Clin. Endocrinol. (Oxf.) 54, 541–546 (2001).
Morita, S. et al. Progressive deterioration of insulin secretion in Japanese type 2 diabetic patients in comparison with those who carry the S20G mutation of the islet amyloid polypeptide gene: a long-term follow-up study. J. Diabetes Investig. 2, 287–292 (2011).
Cao, P. et al. Sensitivity of amyloid formation by human islet amyloid polypeptide to mutations at residue 20. J. Mol. Biol. 421, 282–295 (2012).
Sakagashira, S. et al. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am. J. Pathol. 157, 2101–2109 (2000).
Meier, D. T. et al. The S20G substitution in hIAPP is more amyloidogenic and cytotoxic than wild-type hIAPP in mouse islets. Diabetologia 59, 2166–2171 (2016).
Pilkington, E. H. et al. Pancreatic β-cell membrane fluidity and toxicity induced by human islet amyloid polypeptide species. Sci. Reports 6, 21274 (2016).
Krotee, P. et al. Atomic structures of fibrillar segments of hIAPP suggest tightly mated β-sheets are important for cytotoxicity. Elife 6, e19273 (2017).
Jaikaran, E. T. et al. Identification of a novel human islet amyloid polypeptide beta-sheet domain and factors influencing fibrillogenesis. J. Mol. Biol. 308, 515–525 (2001).
Nilsson, M. R. & Raleigh, D. P. Analysis of amylin cleavage products provides new insights into the amyloidogenic region of human amylin. J. Mol. Biol. 294, 1375–1385 (1999).
Gilead, S. & Gazit, E. The role of the 14–20 domain of the islet amyloid polypeptide in amyloid formation. Exp. Diabetes Res. 2008, 256954 (2008).
Scrocchi, L. A. et al. Identification of minimal peptide sequences in the (8–20) domain of human islet amyloid polypeptide involved in fibrillogenesis. J. Struct. Biol. 141, 218–227 (2003).
Luca, S., Yau, W.-M., Leapman, R. & Tycko, R. Peptide conformation and supramolecular organization in amylin fibrils: constraints from solid-state NMR. Biochemistry 46, 13505–13522 (2007).
Bedrood, S. et al. Fibril structure of human islet amyloid polypeptide. J. Biol. Chem. 287, 5235–5241 (2012).
Cao, Q., Boyer, D. R., Sawaya, M. R., Ge, P. & Eisenberg, D. S. Cryo-EM structures of four polymorphic TDP-43 amyloid cores. Nat. Struct. Mol. Biol. 26, 619–627 (2019).
Guenther, E. L. et al. Atomic structures of TDP-43 LCD segments and insights into reversible or pathogenic aggregation. Nat. Struct. Mol. Biol. 25, 463–471 (2018).
Fitzpatrick, A. W. P. et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547, 185–190 (2017).
Falcon, B. et al. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 561, 137–140 (2018).
Falcon, B. et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568, 420–423 (2019).
Li, B. et al. Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat. Commun. 9, 3609 (2018).
Boyer, D. R. et al. Structures of fibrils formed by α-synuclein hereditary disease mutant H50Q reveal new polymorphs. Nat. Struct. Mol. Biol. 26, 1044–1052 (2019).
Iadanza, M. G. et al. The structure of a β2-microglobulin fibril suggests a molecular basis for its amyloid polymorphism. Nat. Commun. 9, 4517 (2018).
Murray, D. T. et al. Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell 171, 615–627.e16 (2017).
Kollmer, M. et al. Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer’s brain tissue. Nat. Commun. 10, 4760 (2019).
Sakagashira, S. et al. Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes 45, 1279–1281 (1996).
Cao, P., Meng, F., Abedini, A. & Raleigh, D. P. The ability of rodent islet amyloid polypeptide to inhibit amyloid formation by human islet amyloid polypeptide has important implications for the mechanism of amyloid formation and the design of inhibitors. Biochemistry 49, 872–881 (2010).
Akter, R. et al. Islet amyloid polypeptide: structure, function, and pathophysiology. J. Diabetes Res. 2016, 2798269 (2016).
Oskarsson, M. E. et al. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am. J. Pathol. 185, 834–846 (2015).
Janson, J. et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53, 474–481 (2004).
Miklossy, J. et al. Beta amyloid and hyperphosphorylated tau deposits in the pancreas in type 2 diabetes. Neurobiol. Aging 31, 1503–1515 (2010).
Peila, R., Rodriguez, B. L., Launer, L. J. & Honolulu-Asia Aging Study. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 51, 1256–1262 (2002).
Moreno-Gonzalez, I. et al. Molecular interaction between type 2 diabetes and Alzheimer’s disease through cross-seeding of protein misfolding. Mol. Psychiatry 22, 1327–1334 (2017).
O’Nuallain, B., Williams, A. D., Westermark, P. & Wetzel, R. Seeding specificity in amyloid growth induced by heterologous fibrils. J. Biol. Chem. 279, 17490–17499 (2004).
Krotee, P. et al. Common fibrillar spines of amyloid-β and human islet amyloid polypeptide revealed by microelectron diffraction and structure-based inhibitors. J. Biol. Chem. 293, 2888–2902 (2018).
Andreetto, E. et al. A hot-segment-based approach for the design of cross-amyloid interaction surface mimics as inhibitors of amyloid self-assembly. Angew. Chem. Int. Ed. Engl. 54, 13095–13100 (2015).
Sievers, S. A. et al. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. Nature 475, 96–100 (2011).
Seidler, P. M. et al. Structure-based inhibitors of tau aggregation. Nat. Chem. 10, 170–176 (2018).
Seidler, P. M. et al. Structure-based inhibitors halt prion-like seeding by Alzheimer’s disease- and tauopathy-derived brain tissue samples. J. Biol. Chem. 294, 16451–16464 (2019).
Griner, S. L. et al. Structure-based inhibitors of amyloid beta core suggest a common interface with tau. Elife 8, e46924 (2019).
Sangwan, S. et al. Inhibition of synucleinopathic seeding by rationally designed inhibitors. Elife 9, e46775 (2020).
Lopes, D. H. J. et al. Amyloidogenicity and cytotoxicity of recombinant mature human islet amyloid polypeptide (rhIAPP). J. Biol. Chem. 279, 42803–42810 (2004).
Sheng, W. & Liao, X. Solution structure of a yeast ubiquitin-like protein Smt3: the role of structurally less defined sequences in protein–protein recognitions. Protein Sci. 11, 1482–1491 (2002).
Röder, C. et al. Amyloid fibril structure of islet amyloid polypeptide by cryo-electron microscopy reveals similarities with amyloid beta. Preprint at bioRxiv https://doi.org/10.1101/2020.02.11.944546 (2020).
Guenther, E. L. et al. Atomic structures of TDP-43 LCD segments and insights into reversible or pathogenic aggregation. Nat. Struct. Mol. Biol. 25, 463–471 (2018).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Grant, T. & Grigorieff, N. Automatic estimation and correction of anisotropic magnification distortion in electron microscopes. J. Struct. Biol. 192, 204–208 (2015).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Grant, T. & Grigorieff, N. Measuring the optimal exposure for single particle cryo-EM using a 2.6 Å reconstruction of rotavirus VP6. Elife 4, e06980 (2015).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
He, S. & Scheres, S. H. W. Helical reconstruction in RELION. J. Struct. Biol. 198, 163–176 (2017).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Terwilliger, T. C., Sobolev, O. V., Afonine, P. V. & Adams, P. D. Automated map sharpening by maximization of detail and connectivity. Acta Crystallogr. D Struct. Biol. 74, 545–559 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Wiltzius, J. J. W. et al. Atomic structure of the cross-beta spine of islet amyloid polypeptide (amylin). Protein Sci. 17, 1467–1474 (2008).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Eisenberg, D. & McLachlan, A. D. Solvation energy in protein folding and binding. Nature 319, 199–203 (1986).
Eisenberg, D., Wesson, M. & Yamashita, M. Interpretation of protein folding and binding with atomic solvation parameters. Chem. Scr. 29A, 217–221 (1989).
Koehl, P. & Delarue, M. Application of a self-consistent mean field theory to predict protein side-chains conformation and estimate their conformational entropy. J. Mol. Biol. 239, 249–275 (1994).
Alford, R. F. et al. The Rosetta all-atom energy function for macromolecular modeling and design. J. Chem. Theory Comput. 13, 3031–3048 (2017).
Warmack, R. A. et al. Structure of amyloid-beta (20-34) with Alzheimer’s-associated isomerization at Asp23 reveals a distinct protofilament interface. Nat. Commun. 10, 3357 (2019).
Lu, J.-X. et al. Molecular structure of beta-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154, 1257–1268 (2013).
Schutz, A. K. et al. Atomic-resolution three-dimensional structure of amyloid beta fibrils bearing the Osaka mutation. Angew. Chem. Int. Ed. Engl. 54, 331–335 (2015).
Colvin, M. T. et al. Atomic resolution structure of monomorphic Aβ42 amyloid fibrils. J. Am. Chem. Soc. 138, 9663–9674 (2016).
Gremer, L. et al. Fibril structure of amyloid-β(1–42) by cryo–electron microscopy. Science 358, 116–119 (2017).
Walti, M. A. et al. Atomic-resolution structure of a disease-relevant Aβ(1–42) amyloid fibril. Proc. Natl Acad. Sci. USA 113, E4976–E4984 (2016).
Xiao, Y. et al. Aβ(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 22, 499–505 (2015).
Luhrs, T. et al. 3D structure of Alzheimer’s amyloid-β(1–42) fibrils. Proc. Natl Acad. Sci. USA 102, 17342–17347 (2005).
Paravastu, A. K., Leapman, R. D., Yau, W.-M. & Tycko, R. Molecular structural basis for polymorphism in Alzheimer’s β-amyloid fibrils. Proc. Natl Acad. Sci. USA 105, 18349–18354 (2008).
Sgourakis, N. G., Yau, W.-M. & Qiang, W. Modeling an in-register, parallel “Iowa” Aβ fibril structure using solid-state NMR data from labeled samples with Rosetta. Structure 23, 216–227 (2015).
Brunger, A. T. Version 1.2 of the Crystallography and NMR system. Nat. Protoc. 2, 2728–2733 (2007).
Acknowledgements
We thank H. Zhou for the use of Electron Imaging Center for Nanomachines (EICN) resources. We acknowledge the use of instruments at the EICN supported by the NIH (1S10RR23057 and IS10OD018111), NSF (DBI-1338135) and CNSI at UCLA. The authors acknowledge NIH AG 054022, NIH AG061847, and DOE DE-FC02-02ER63421 for support. D.R.B. was supported by the National Science Foundation Graduate Research Fellowship Program.
Author information
Authors and Affiliations
Contributions
Q.C. designed experiments, purified constructs, prepared cryo-EM samples, performed cryo-EM data collection and processing, designed inhibitors, performed biochemical experiments and performed data analysis. D.R.B. and P.G. assisted in cryo-EM data collection and processing. Q.C. and M.R.S. built the inhibitor binding model. M.R.S. performed solvation energy calculation. All authors analyzed the results and wrote the manuscript. D.S.E. supervised and guided the project.
Corresponding author
Ethics declarations
Competing interests
D.S.E. is an advisor and equity shareholder in ADRx, Inc.
Additional information
Peer review information Peer reviewer reports are available. Inês Chen was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM data processing.
a, Representative Krios micrograph of hIAPP fibrils. Blue and green arrows indicate two morphologies (twister and ribbon, respectively) identified by 2D classification. Notice they are not distinguishable by eye. b, Representative 2D classes and relative population of twister and ribbon morphologies. c, Representative 2D classes of twister with smaller box size particles showing the 4.8 Å β-sheet spacing, and the computed diffraction pattern from a representative 2D class. d, Central slice (left) and 2D projection (right) from the final reconstruction. Notice the 2D projection of final reconstruction is consistent with 2D classification. e-f, FSC curves between two half-maps (e) and the cryo-EM reconstruction and refined atomic model (f). Data for graphs in e and f are available as source data.
Extended Data Fig. 2 Potential domain swapping of hIAPP models.
Domain swapped versions of both Model 1 and Model 2 were built to test the possibility of domain swapping. In the swapped models, the residues between the N-terminus and Gly24 from one protofilament were connected to the residues between Ala25 and the C-terminus from the other protofilament of the un-swapped model. The density map with σ=3.0 is shown in blue mesh and that with σ=2.0 is shown in grey mesh. Notice that Gly24 in both swapped Model 1 and Model 2 is clearly out of the density, demonstrating that the domain swapping is not supported by our cryo-EM map.
Extended Data Fig. 3 The fuzzy coat in hIAPP fibril structure may represent the flexible N-terminal of hIAPP and the SUMO-tag.
a, The final reconstruction (left) and 2D classification (right) show a fuzzy coat of ~55 Å surrounding the fibril core. b, Protease cleavage assays indicate the construct we used for fibril structure determination (SUMO-IAPP with 1xG, means one glycine between SUMO-tag and hIAPP) has an un-removable SUMO-tag, whereas the SUMO-tag is removable when we extend the linker to three glycine. c, Plausible N-terminal conformation suggested by the extra densities near Asn14. The density map with σ=3.0 is shown in blue mesh and that with σ=2.0 is shown in grey mesh. The intra-molecular disulfide bond is labeled between Cys2 and Cys7, and the residues occupying the extra densities in our hypothetical model are underlined. d, Crystal structure of SUMO protein (PDB ID 1L2N). e, Hypothetical model of N-terminus of hIAPP and SUMO-tag match the dimensions of the fuzzy coat observed in the hIAPP fibril reconstruction. Notice that in most cases the SUMO-tag is far away from the fibril core therefore should not influence the fibril structure.
Extended Data Fig. 4 Rosetta energy minimization of hIAPP fibril structure and rIAPP homology model.
a, Structure superimposition between (grey) hIAPP fibril structure determined here and (blue) hIAPP fibril structure (upper panels) or rIAPP homology model (lower panels) optimized by Rosetta energy minimization. Calculation was done either allowing only side chain movements (left panels) or allowing both side chain and main chain movements (middle and right panels). Notice that during Rosetta energy minimization, we did not apply non-crystallographic symmetry so that the 5 layers in each model were not forced to be identical. b, Steric clashes of the rIAPP homology model after side chain Rosetta energy minimization were probed with COOT and displayed as red dots. Notice that most of the steric clashes are found near S28P and S29P.
Extended Data Fig. 5 Structural superimposition of Aβ fibril structures and hIAPP fibril structure.
Ten previously reported Aβ fibril structures were superimposed with the hIAPP fibril structure by either directly comparing full-length Aβ fibril structures with the full-length hIAPP structure, or by only comparing residues 24-34 of Aβ fibril structures with residues 19-29 of the hIAPP structure. For the full-length comparison, one Aβ fibril structure (PDB ID 6SHS) shows reasonable alignment with low r.m.s.d., and the structural superimposition is shown on the far left panel, with the Aβ fibril structure shown in grey, the hIAPP structure shown in blue, and the segment that fits best (residues 20-25 of Aβ fibril structure) shown in magenta. For the partial comparison, four Aβ fibril structures show a good fit (middle left), three Aβ fibril structures show a moderate fit (middle right) and four Aβ fibril structures do not fit (far right). In these superimpositions, residues 24-34 of the Aβ fibril structures were colored grey and the highest fitting region (residues 26-31) is colored magenta. Detailed alignment parameters are listed in Supplementary Table 3.
Extended Data Fig. 6 Segments selected for hIAPP fibril inhibitor design.
Three segments of hIAPP, 21NNFGAILSS29 (N9S, left panels), 25AILSSTNVG33 (A9G, middle panels) and 21NNFG24 (N4G, right panels), were selected for design of inhibitors of hIAPP fibrils. For each selected segment, the hIAPP structure with the segment highlighted is shown on the top, with the hIAPP structure shown as lines and the segment shown as sticks. Proposed models of the corresponding inhibitor peptides (before adding N-methylation) binding to the hIAPP structures are shown as top views (middle panels) and side views (bottom panels). Notice there are multiple hydrogen bonds between the designed inhibitors and hIAPP fibrils, providing binding affinities for these inhibitors. For the N4G merged inhibitor, the model indicates the orientation-flipped and chirality-reversed N4G has high structural similarity to the original N4G and recaptures all original inter-layer interactions. Hydrogen bonds with distances between 2.3-3.2 Å are shown as black dashed lines.
Extended Data Fig. 7 Additional inhibitors designed for hIAPP fibrils.
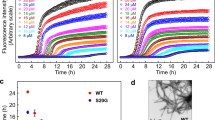
a, Proposed model of designed inhibitors (magenta) bound to hIAPP fibrils (blue and grey for each protofilament). The methyl group of N-methylated inhibitors is shown as a green sphere. The last three residues of N4Gm-A are d-amino acids and are underlined. b, ThT assays measuring inhibitor efficacy shown on the left. A9G-A delays hIAPP fibril formation but not N9S-B and N4Gm-A. For the two inhibitors that were not effective, two effective inhibitors (N9S-A and N4Gm-B, respectively) are tested in the same experiment as controls. Data are shown as mean ± s.d., n = 3 independent experiments. c, Negative stain EM images of hIAPP with N9S-A, A9G-A or A9G-B after 20 hours of incubation. Notice that hIAPP fibril formation is not fully eliminated by these inhibitors. d, Negative stain EM shows hIAPP S20G fibrils present after 3 days of incubation with N4Gm-B, suggesting that fibril formation of hIAPP S20G is not fully eliminated when longer incubation times are examined (compared to 20 hours shown in Fig. 3e). Data for graphs in b are available as source data.
Extended Data Fig. 8 Connection of N-terminal density.
Slices of 3D maps of the final reconstruction (left) and an earlier reconstruction with lower resolution (right). The positions that represent N-terminus of Model 1 and Model 2 are indicated by arrows. Note the weak density that represents the flexible N-terminus of hIAPP seems to connect to the position that represents the N-terminus of Model 2 in the final reconstruction (left); whereas in the lower resolution reconstruction (right), the weak density seems to connect to the position of N-terminus of Model 1.
Supplementary information
Supplementary Information
Supplementary Tables 1−4 and Supplementary Note 1.
Supplementary Data 1
Coordinates of model 2.
Supplementary Data 2
Coordinates of model 1 (swap).
Supplementary Data 3
Coordinates of model 2 (swap).
Source data
Source Data Fig. 3
Statistical source data for Fig.3b,d
Source Data Extended Data Fig. 1
Statistical source data for Extended Data Fig. 1e,f
Source Data Extended Data Fig. 7
Statistical source data for Extended Data Fig. 7b
Rights and permissions
About this article
Cite this article
Cao, Q., Boyer, D.R., Sawaya, M.R. et al. Cryo-EM structure and inhibitor design of human IAPP (amylin) fibrils. Nat Struct Mol Biol 27, 653–659 (2020). https://doi.org/10.1038/s41594-020-0435-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-020-0435-3
This article is cited by
-
Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases
Molecular Neurodegeneration (2024)
-
Network of hotspot interactions cluster tau amyloid folds
Nature Communications (2023)
-
Mechanisms and pathology of protein misfolding and aggregation
Nature Reviews Molecular Cell Biology (2023)
-
Islet amyloid polypeptide cross-seeds tau and drives the neurofibrillary pathology in Alzheimer’s disease
Molecular Neurodegeneration (2022)
-
Mapping the sequence specificity of heterotypic amyloid interactions enables the identification of aggregation modifiers
Nature Communications (2022)