Abstract
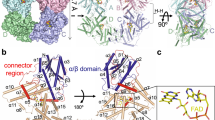
Cryptochromes (CRYs) are a group of evolutionarily conserved flavoproteins found in many organisms. In plants, the well-studied CRY photoreceptor, activated by blue light, plays essential roles in plant growth and development. However, the mechanism of activation remains largely unknown. Here, we determined the oligomeric structures of the blue-light-perceiving PHR domain of Zea mays CRY1 and an Arabidopsis CRY2 constitutively active mutant. The structures form dimers and tetramers whose functional importance is examined in vitro and in vivo with Arabidopsis CRY2. Structure-based analysis suggests that blue light may be perceived by CRY to cause conformational changes, whose precise nature remains to be determined, leading to oligomerization that is essential for downstream signaling. This photoactivation mechanism may be widely used by plant CRYs. Our study reveals a molecular mechanism of plant CRY activation and also paves the way for design of CRY as a more efficient optical switch.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The cryo-EM maps of ZmCRY1cW368A-PHR and AtCRY2W374A-PHR tetramers (3.2 Å and 4.2 Å) and dimers (7.2 Å and 5.6 Å) have been deposited in the EMDB with accession codes EMD-30022, EMD-30023, EMD-30024 and EMD-30025. The atomic coordinates of ZmCRY1cW368A-PHR and ZmCRY1a-PHR structures have been deposited in the Protein Data Bank with accession codes 6LZ3 and 6LZ7, respectively. Source data for Figs. 1a and 4e,f are available with the paper online.
Change history
18 May 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41594-020-0449-x
References
Emery, P., So, W. V., Kaneko, M., Hall, J. C. & Rosbash, M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95, 669–679 (1998).
Stanewsky, R. et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95, 681–692 (1998).
Griffin, E. A. J., Staknis, D. & Weitz, C. J. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286, 768–771 (1999).
Kume, K. et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205 (1999).
van der Horst, G. T. et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630 (1999).
Chaves, I. et al. The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62, 335–364 (2011).
Lin, C. & Shalitin, D. Cryptochrome structure and signal transduction. Annu. Rev. Plant. Biol. 54, 469–496 (2003).
Cashmore, A. R. Cryptochromes. Cell 114, 537–543 (2003).
Cashmore, A. R., Jarillo, J. A., Wu, Y. J. & Liu, D. Cryptochromes: blue light receptors for plants and animals. Science 284, 760–765 (1999).
Ahmad, M. & Cashmore, A. R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366, 162–166 (1993).
Guo, H., Yang, H., Mockler, T. C. & Lin, C. Regulation of flowering time by Arabidopsis photoreceptors. Science 279, 1360–1363 (1998).
Kleine, T., Lockhart, P. & Batschauer, A. An Arabidopsis protein closely related to Synechocystis cryptochrome is targeted to organelles. Plant J. 35, 93–103 (2003).
Ahmad, M., Jarillo, J. A. & Cashmore, A. R. Chimeric proteins between cry1 and cry2 Arabidopsis blue light photoreceptors indicate overlapping functions and varying protein stability. Plant Cell. 10, 197–207 (1998).
Lin, C. et al. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl Acad. Sci. USA 95, 2686–2690 (1998).
Wang, H., Ma, L. G., Li, J. M., Zhao, H. Y. & Deng, X. W. Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294, 154–158 (2001).
Yang, H. Q., Tang, R. H. & Cashmore, A. R. The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13, 2573–2587 (2001).
Lian, H. L. et al. Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev. 25, 1023–1028 (2011).
Zuo, Z., Liu, H., Liu, B., Liu, X. & Lin, C. Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr. Biol. 21, 841–847 (2011).
Liu, H. T. et al. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322, 1535–1539 (2008).
Wang, W. et al. Photoexcited CRYPTOCHROME1 interacts with dephosphorylated BES1 to regulate brassinosteroid signaling and photomorphogenesis in Arabidopsis. Plant Cell 30, 1989–2005 (2018).
Pedmale, U. V. et al. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 164, 233–245 (2016).
Ma, D. et al. Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl Acad. Sci. USA 113, 224–229 (2016).
He, S. B. et al. The CNT1 domain of Arabidopsis CRY1 alone is sufficient to mediate blue light inhibition of hypocotyl elongation. Mol. Plant 8, 822–825 (2015).
Wang, Q. et al. Photoactivation and inactivation of Arabidopsis cryptochrome 2. Science 354, 343–347 (2016).
Yang, H. Q. et al. The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 103, 815–827 (2000).
Shalitin, D. et al. Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 417, 763–767 (2002).
Sang, Y. et al. N-terminal domain-mediated homodimerization is required for photoreceptor activity of Arabidopsis CRYPTOCHROME 1. Plant Cell 17, 1569–1584 (2005).
Yu, X. et al. Derepression of the NC80 motif is critical for the photoactivation of Arabidopsis CRY2. Proc. Natl Acad. Sci. USA 104, 7289–7294 (2007).
Engelhard, C. et al. Cellular metabolites enhance the light sensitivity of Arabidopsis cryptochrome through alternate electron transfer pathways. Plant Cell 26, 4519–4531 (2014).
Li, X. et al. Arabidopsis cryptochrome 2 (CRY2) functions by the photoactivation mechanism distinct from the tryptophan (Trp) triad-dependent photoreduction. Proc. Natl Acad. Sci. USA 108, 20844–20849 (2011).
Wang, Q. et al. Beyond the photocycle — how cryptochromes regulate photoresponses in plants? Curr. Opin. Plant Biol. 45, 120–126 (2018).
Park, H. W., Kim, S. T., Sancar, A. & J, D. Crystal structure of DNA photolyase from Escherichia coli. Science 268, 1866–1872 (1995).
Brautigam, C. A. et al. Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 101, 12142–12147 (2004).
Huang, Y. et al. Crystal structure of cryptochrome 3 from Arabidopsis thaliana and its implications for photolyase activity. Proc. Natl Acad. Sci. USA 103, 17701–17706 (2006).
Aubert, C., Vos, M. H., Mathis, P., Eker, A. P. & Brettel, K. Intraprotein radical transfer during photoactivation of DNA photolyase. Nature 405, 586–590 (2000).
Giovani, B., Byrdin, M., Ahmad, M. & Brettel, K. Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nat. Struct. Biol. 10, 489–490 (2003).
Zeugner, A. et al. Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function. J. Biol. Chem. 280, 19437–19440 (2005).
Gao, J. et al. Trp triad-dependent rapid photoreduction is not required for the function of Arabidopsis CRY1. Proc. Natl Acad. Sci. USA 112, 9135–9140 (2015).
Taslimi, A. et al. Optimized second-generation CRY2-CIB dimerizers and photoactivatable Cre recombinase. Nat. Chem. Biol. 12, 425–430 (2016).
Partch, C. L., Clarkson, M. W., Özgür, S., Lee, A. L. & Sancar, A. Role of structural plasticity in signal transduction by the cryptochrome blue-light photoreceptor. Biochemistry 44, 3795–3805 (2005).
Kondoh, M. & Terazima, M. Conformational and intermolecular interaction dynamics of photolyase/cryptochrome proteins monitored by the time-resolved diffusion technique. Photochem. Photobiol. 93, 15–25 (2017).
Shalitin, D., Yu, X., Maymon, M., Mockler, T. & Lin, C. Blue light-dependent in vivo and in vitro phosphorylation of Arabidopsis cryptochrome 1. Plant Cell 15, 2421–2429 (2003).
Wang, Q. et al. The blue light-dependent phosphorylation of the CCE domain determines the photosensitivity of Arabidopsis CRY2. Mol. Plant 10, 357 (2017).
Liu, Q. et al. Molecular basis for blue light-dependent phosphorylation of Arabidopsis cryptochrome 2. Nat. Commun. 8, 15234 (2017).
El-Din El-Assal, S., Alonso-Blanco, C., Peeters, A. J., Raz, V. & Koornneef, M. A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat. Genet. 29, 435–440 (2001).
Gu, N. N., Zhang, Y. C. & Yang, H. Q. Substitution of a conserved glycine in the PHR domain of Arabidopsis cryptochrome 1 confers a constitutive light response. Mol. Plant 5, 85–97 (2012).
Schmalen, I. et al. Interaction of circadian clock proteins CRY1 and PER2 is modulated by zinc binding and disulfide bond formation. Cell 157, 1203–1215 (2014).
Xing, W. et al. SCF(FBXL3) ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature 496, 64–68 (2013).
Czarna, A. et al. Structures of Drosophila cryptochrome and mouse cryptochrome1 provide insight into circadian function. Cell 153, 1394–1405 (2013).
Zoltowski, B. D. et al. Structure of full-length Drosophila cryptochrome. Nature 480, 396–399 (2011).
Bugaj, L. J., Choksi, A. T., Mesuda, C. K., Kane, R. S. & Schaffer, D. V. Optogenetic protein clustering and signaling activation in mammalian cells. Nat. Methods 10, 249–252 (2013).
Zhang, K. & Cui, B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 33, 92–100 (2015).
Duan, L. et al. Understanding CRY2 interactions for optical control of intracellular signaling. Nat. Commun. 8, 547 (2017).
Lei, J. & Frank, J. Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. J. Struct. Biol. 150, 69–80 (2005).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Grant, T., Rohou, A. & Grigorieff, N. cisTEM, user-friendly software for single-particle image processing. Elife 7, e35383 (2018).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & K, C. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol. Crystallogr. 66, 213–221 (2010).
Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. HKL-3000: the integration of data reduction and structure solution-from diffraction images to an initial model in minutes. Acta Crystallogr D Biol. Crystallogr. 62, 859–866 (2006).
Acknowledgements
We thank the staff members at the cryo-EM centers of Tsinghua University (L. Zhao & H. Wang), Zhejiang University and the National Facility for Protein Science in Shanghai Zhangjiang Lab for their technical assistance on cryo-EM image and SEC-MALLS data collection and analysis. We also thank the staff members at BL19U1 for their technical assistance in X-ray diffraction data collection, and the core facility center of the Institute of Plant Physiology and Ecology for X-ray diffraction testing. This work was supported by grants from the National Key R&D Program of China (SQ2018YFA090071 to P.Z. and F.Y.), the Chinese Academy of Sciences (CAS) (XDB27020103 and QYZDB-SSW-SMC006 to P.Z., and XDB27030000 to H.L.), the National Natural Science Foundation of China (31870727 to M.Z., 31861130356 to P.Z. and 31825004 to H.L.) and the Shanghai Science and technology Commission (19XD1424500 to P.Z.). Dr. X.L. is supported by the foundation of Youth Innovation Promotion Association of CAS.
Author information
Authors and Affiliations
Contributions
K.S. carried out protein expression, purification, ITC, SEC-MALLS analysis, crystallization, and cryo-EM sample preparation; X.Z. carried out cryo-EM data collection and structure determination; X.L. carried out the physiological analysis of AtCRY2 and mutants; M.M., Y.H. and F.Y. contributed to protein expression, purification, crystallization and ITC analysis; M.Z., and X.H. contributed to X-ray and cryo-EM data collection and structure determination. P.Z. and H.L. analyzed the data and wrote the manuscript with inputs from other authors. P.Z. conceived the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editor recognition statement: Beth Moorefield was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of AtCRY2 and constitutively active/inactive mutants AtCRY2W374A/AtCRY2D387A.
a, Purified protein samples were resolved by SDS-PAGE and visualized by Coomassie blue staining. b, Color view of samples from A. c, The absorption spectra of AtCRY2 and mutants under daylight (daylight was not sufficient to induce light-activation). a.u., arbitrary units.
Extended Data Fig. 2 Size Exclusion Chromatography-Multi Angle Laser Light Scattering (SEC-MALLS) analysis of wild-type and mutant CRYs.
Representative SEC-MALLS analysis of AtCRY2, AtCRY2W374A, ZmCRY1c, ZmCRY1cW368A, AtCRY2W374AW349A, AtCRY2W374AW439A, AtCRY2D387A, respectively. Aggregates were observed in AtCRY2, and double mutants AtCRY2W374AW349A and AtCRY2W374AW439A (eluting between 8–10 ml). The chromatogram shows readings of the UV and RI detectors in blue and red, respectively. The green/yellow/black dotted lines indicate the calculated molecular mass of the protein samples eluted from a Superdex-200 10/300 column. The theoretical molecular weight of each protein is shown in parentheses.
Extended Data Fig. 3 Isothermal calorimetry titration curves of AtCRY and mutants with AtBIC116–43 peptide.
The constitutively active mutant AtCRY2W374A shows strong binding with AtCIB116–43, while wild type AtCRY2, inactive mutant AtCRY2 D387A and dimer interface mutants AtCRY2W374A/W349A and AtCRY2W374A/R439L show no significant binding with AtCIB116–43. AtCRY2W374A/W349A and AtCRY2W374A/R439L were selected for ITC binding affinity measurements because either of them could lead to the promote dissociation of AtCRYW374A dimer to a monomer.
Extended Data Fig. 4 Cryo-EM images, 2D averages and 3D reconstruction of AtCRY2W374A-PHR.
a, Representative cryo-EM micrograph of AtCRY2W374A. b-c, Representative images of the 2D class averages of tetramer and dimer, respectively. d, FSC curves at 0.5 and 0.143 of the final reconstruction of AtCRY2W374A-PHR tetramer and dimer. e, Local resolution estimation of the final sharpened cryo-EM density map of AtCRY2W374A-PHR tetramer.
Extended Data Fig. 5 Purification and structure reconstruction of ZmCRY1cW368A.
a, Purification profiles of ZmCRY1cW368A and ZmCRY1c. Protein samples of ZmCRY1c and ZmCRY1cW368A were applied to Superdex-200 10/300, and the peak fractions of ZmCRY1cW368A were visualized by SDS-PAGE and Coomassie blue staining. Molecular weights are labelled according to the SEC-MALLS results. b, Flowchart of cryo-EM data processing and 3D reconstruction of ZmCRY1cW368A. c, FSC curves at 0.5 and 0.143 of the final reconstruction of ZmCRY1cW368A-PHR. d, Local resolution estimation of the final sharpened cryo-EM density map of ZmCRY1cW368A-PHR tetramer.
Extended Data Fig. 6 Superimposition of the cryo-EM maps and structures.
a, Different views of cryo-EM map of ZmCRY1cW368A-PHR dimer (blue) superimposed to that of ZmCRY1cW368A-PHR tetramer (light gray). b, Side views of the cryo-EM density of AtCRY2W374A-PHR tetramer fitted with cryo-EM structure of ZmCRY1cW368A-PHR tetramer. c, Side and front views of the cryo-EM density of AtCRY2W374A-PHR dimer fitted with cryo-EM structure of ZmCRY1cW368A-PHR dimer. d, Superimposition of the tetrameric structures of ZmCRY1cW368A-PHR and ZmCRY1a-PHR. ZmCRY1cW368A-PHR tetramer structure molecules are colored in gray and ZmCRY1a-PHR crystal structure molecules are colored in yellow, magenta, orange and cyan.
Extended Data Fig. 7 Ribbon cartoon of ZmCRY1cW368A shows the residues interacting with FAD.
FAD is shown as a yellow stick model. Residues interacting with FAD are shown with side chains. Those residues that are conserved among CRYs from different organisms and with photolyases are colored in magenta, while those that are conserved only within plants are colored green.
Extended Data Fig. 8 Protein sequence alignment of cryptochromes-PHR and photolyases.
Protein sequences of cryptochromes-PHR and photolyases from different species are aligned. The secondary structure elements of ZmCRY1cW368A-PHR and mCRY1-PHR are indicated at the top and bottom, respectively. Protein sequences are separated by dashed lines. Regions constituting INT1 and INT2 are indicated with blue and green solid lines respectively. Highly conserved residues are shaded red, while less conserved residues are red colored and boxed. Note that specific residues from helices α6, α7, α13 and α18-α19 loop at INT1 are colored blue. Residues interacting with FAD are indicated with yellow and green triangles; the latter indicate those residues that are conserved only within plants. Species are: Zm, Zea mays; At, Arabidopsis thaliana; Os, Oryza sativa; Gm, Glycine max; mCRY, mouse CRY; dCRY, Drosophila CRY; photolyase, Escherichia coli photolyase.
Extended Data Fig. 9 Dimer interface 2 and locations of CRY mutations in ZmCRY1cW368A-PHR structure.
a, dimer interface formed by molecules A/C (or B/D). Mol A is shown as a surface model, while C is shown as a ribbon model. FAD is depicted as yellow sticks, and the “trp-triad” residues are shown as orange spheres. b, The reported CRY mutations were located in the ZmCRY1cW368A-PHR structure. The early-flowering mutation AtCRY2V367M in the Cvi nature allele and the constitutive COP-phenotype mutation AtCRY1G380R corresponds to residues Val361 and Gly371 in ZmCRY1cW368A-PHR, respectively. Their locations near the “trp-triad”residues are shown in the structure.
Extended Data Fig. 10 Sequence alignment of ZmCRY1c, ZmCRY1a, AtCRY1, and AtCRY2 at “trp-triad” residues and dimer interaction residues.
The “trp-triad” residues and dimer interaction residues are shaded in blue and magenta, respectively. The numbers of corresponding residues are indicated.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed gel image for Fig.1a, lower panel.
Source Data Fig. 2
Unprocessed western blot image for Fig.4e.
Source Data Fig. 3
Unprocessed western blot image for Fig.4f.
Rights and permissions
About this article
Cite this article
Shao, K., Zhang, X., Li, X. et al. The oligomeric structures of plant cryptochromes. Nat Struct Mol Biol 27, 480–488 (2020). https://doi.org/10.1038/s41594-020-0420-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-020-0420-x
This article is cited by
-
A new family of proteins is required for tethering of Casparian strip membrane domain and nutrient homoeostasis in rice
Nature Plants (2023)
-
Photoexcited cryptochromes interact with ADA2b and SMC5 to promote the repair of DNA double-strand breaks in Arabidopsis
Nature Plants (2023)
-
A marine cryptochrome with an inverse photo-oligomerization mechanism
Nature Communications (2023)
-
Light-induced LLPS of the CRY2/SPA1/FIO1 complex regulating mRNA methylation and chlorophyll homeostasis in Arabidopsis
Nature Plants (2023)
-
Common evolutionary trajectory of short life-cycle in Brassicaceae ruderal weeds
Nature Communications (2023)