Abstract
Leading-strand template aberrations cause helicase–polymerase uncoupling and impede replication fork progression, but the details of how uncoupled forks are restarted remain uncertain. Using purified proteins from Saccharomyces cerevisiae, we have reconstituted translesion synthesis (TLS)-mediated restart of a eukaryotic replisome following collision with a cyclobutane pyrimidine dimer. We find that TLS functions ‘on the fly’ to promote resumption of rapid replication fork rates, despite lesion bypass occurring uncoupled from the Cdc45-MCM-GINS (CMG) helicase. Surprisingly, the main lagging-strand polymerase, Pol δ, binds the leading strand upon uncoupling and inhibits TLS. Pol δ is also crucial for efficient recoupling of leading-strand synthesis to CMG following lesion bypass. Proliferating cell nuclear antigen monoubiquitination positively regulates TLS to overcome Pol δ inhibition. We reveal that these mechanisms of negative and positive regulation also operate on the lagging strand. Our observations have implications for both fork restart and the division of labor during leading-strand synthesis generally.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are provided in full in the Results section and the Supplementary Information accompanying this paper. Unprocessed gels are available with the paper online.
References
Daigaku, Y. et al. A global profile of replicative polymerase usage. Nat. Struct. Mol. Biol. 22, 192–198 (2015).
McElhinny, N. S. A., Gordenin, D. A., Stith, C. M., Burgers, P. M. J. & Kunkel, T. A. Division of labor at the eukaryotic replication fork. Mol. Cell 30, 137–144 (2008).
Pursell, Z. F., Isoz, I., Lundström, E.-B., Johansson, E. & Kunkel, T. A. Yeast DNA polymerase ε participates in leading-strand DNA replication. Science 317, 127–130 (2007).
Zeman, M. K. & Cimprich, K. A. Causes and consequences of replication stress. Nat. Cell Biol. 16, 2–9 (2014).
Marians, K. J. Lesion bypass and the reactivation of stalled replication forks. Annu. Rev. Biochem. 87, 217–238 (2018).
Sale, J. E., Lehmann, A. R. & Woodgate, R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat. Rev. Mol. Cell Biol. 13, 141–152 (2012).
McCulloch, S. D. & Kunkel, T. A. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 18, 148–161 (2008).
Leung, W., Baxley, R. M., Moldovan, G.-L. & Bielinsky, A.-K. Mechanisms of DNA damage tolerance: post-translational regulation of PCNA. Genes 10, 10 (2019).
Bienko, M. et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310, 1821–1824 (2005).
Plosky, B. S. et al. Controlling the subcellular localization of DNA polymerases ι and η via interactions with ubiquitin. EMBO J. 25, 2847–2855 (2006).
Hoege, C., Pfander, B., Moldovan, G.-L., Pyrowolakis, G. & Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419, 135–141 (2002).
Stelter, P. & Ulrich, H. D. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425, 188–191 (2003).
Garg, P. & Burgers, P. M. Ubiquitinated proliferating cell nuclear antigen activates translesion DNA polymerases η and REV1. Proc. Natl Acad. Sci. USA 102, 18361–18366 (2005).
Haracska, L., Unk, I., Prakash, L. & Prakash, S. Ubiquitylation of yeast proliferating cell nuclear antigen and its implications for translesion DNA synthesis. Proc. Natl Acad. Sci. USA 103, 6477–6482 (2006).
Acharya, N. et al. Roles of PCNA-binding and ubiquitin-binding domains in human DNA polymerase η in translesion DNA synthesis. Proc. Natl Acad. Sci. USA 105, 17724–17729 (2008).
Acharya, N., Brahma, A., Haracska, L., Prakash, L. & Prakash, S. Mutations in the ubiquitin binding UBZ motif of DNA polymerase η do not impair its function in translesion synthesis during replication. Mol. Cell. Biol. 27, 7266–7272 (2007).
Parker, J. L., Bielen, A. B., Dikic, I. & Ulrich, H. D. Contributions of ubiquitin- and PCNA-binding domains to the activity of polymerase η in Saccharomyces cerevisiae. Nucleic Acids Res. 35, 881–889 (2007).
Acharya, N. et al. Reply to Sabbioneda et al.: role of ubiquitin-binding motif of human DNA polymerase η in translesion synthesis. Proc. Natl Acad. Sci. USA 106, E21 (2009).
Arakawa, H. et al. A role for PCNA ubiquitination in immunoglobulin hypermutation. PLoS Biol. 4, e366 (2006).
Despras, E., Delrieu, N., Garandeau, C., Ahmed‐Seghir, S. & Kannouche, P. L. Regulation of the specialized DNA polymerase η: revisiting the biological relevance of its PCNA- and ubiquitin-binding motifs. Environ. Mol. Mutagen. 53, 752–765 (2012).
Göhler, T., Sabbioneda, S., Green, C. M. & Lehmann, A. R. ATR-mediated phosphorylation of DNA polymerase η is needed for efficient recovery from UV damage. J. Cell Biol. 192, 219–227 (2011).
Hendel, A. et al. PCNA ubiquitination is important, but not essential for translesion DNA synthesis in mammalian cells. PLoS Genet. 7, e1002262 (2011).
Krijger, P. H. L. et al. PCNA ubiquitination-independent activation of polymerase η during somatic hypermutation and DNA damage tolerance. DNA Repair 10, 1051–1059 (2011).
Sabbioneda, S. et al. Effect of proliferating cell nuclear antigen ubiquitination and chromatin structure on the dynamic properties of the Y-family DNA polymerases. Mol. Biol. Cell 19, 5193–5202 (2008).
Edmunds, C. E., Simpson, L. J. & Sale, J. E. PCNA ubiquitination and REV1 define temporally distinct mechanisms for controlling translesion synthesis in the avian cell line DT40. Mol. Cell 30, 519–529 (2008).
Lopes, M., Foiani, M. & Sogo, J. M. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell 21, 15–27 (2006).
Daigaku, Y., Davies, A. A. & Ulrich, H. D. Ubiquitin-dependent DNA damage bypass is separable from genome replication. Nature 465, 951–955 (2010).
Karras, G. I. & Jentsch, S. The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S phase. Cell 141, 255–267 (2010).
Yeeles, J. T. P., Janska, A., Early, A. & Diffley, J. F. X. How the eukaryotic replisome achieves rapid and efficient DNA replication. Mol. Cell 65, 105–116 (2017).
Yeeles, J. T. P., Deegan, T. D., Janska, A., Early, A. & Diffley, J. F. X. Regulated eukaryotic DNA replication origin firing with purified proteins. Nature 519, 431–435 (2015).
Taylor, M. R. G. & Yeeles, J. T. P. Dynamics of replication fork progression following helicase–polymerase uncoupling in eukaryotes. J. Mol. Biol. 431, 2040–2049 (2019).
Taylor, M. R. G. & Yeeles, J. T. P. The initial response of a eukaryotic replisome to DNA damage. Mol. Cell 70, 1067–1080 (2018).
Devbhandari, S., Jiang, J., Kumar, C., Whitehouse, I. & Remus, D. Chromatin constrains the initiation and elongation of DNA replication. Mol. Cell 65, 131–141 (2017).
Sparks, J. L. et al. The CMG helicase bypasses DNA–protein cross-links to facilitate their repair. Cell 176, 167–181 (2019).
Aria, V. & Yeeles, J. T. P. Mechanism of bidirectional leading-strand synthesis establishment at eukaryotic DNA replication origins. Mol. Cell 73, 199–211 (2019).
Garbacz, M. A. et al. Evidence that DNA polymerase δ contributes to initiating leading strand DNA replication in Saccharomyces cerevisiae. Nat. Commun. 9, 858 (2018).
Zhou, Z.-X., Lujan, S. A., Burkholder, A. B., Garbacz, M. A. & Kunkel, T. A. Roles for DNA polymerase δ in initiating and terminating leading strand DNA replication. Nat. Commun. 10, 3992 (2019).
Becker, J. R. et al. Genetic interactions implicating postreplicative repair in Okazaki fragment processing. PLoS Genet. 11, e1005659 (2015).
Davies, A. A., Huttner, D., Daigaku, Y., Chen, S. & Ulrich, H. D. Activation of ubiquitin-dependent DNA damage bypass is mediated by replication protein A. Mol. Cell 29, 625–636 (2008).
Watanabe, K. et al. Rad18 guides polη to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 23, 3886–3896 (2004).
Toledo, L., Neelsen, K. J. & Lukas, J. Replication catastrophe: when a checkpoint fails because of exhaustion. Mol. Cell 66, 735–749 (2017).
Toledo, L. I. et al. ATR prohibits replication catastrophe by preventing global exhaustion of RPA. Cell 155, 1088–1103 (2013).
Guilliam, T. A. et al. Molecular basis for PrimPol recruitment to replication forks by RPA. Nat. Commun. 8, 15222 (2017).
Guilliam, T. A. & Doherty, A. J. PrimPol—prime time to reprime. Genes 8, 20 (2017).
Georgescu, R. E. et al. Mechanism of asymmetric polymerase assembly at the eukaryotic replication fork. Nat. Struct. Mol. Biol. 21, 664–670 (2014).
Georgescu, R. et al. Structure of eukaryotic CMG helicase at a replication fork and implications to replisome architecture and origin initiation. Proc. Natl Acad. Sci. USA 114, E697–E706 (2017).
Zhou, J. C. et al. CMG–Pol epsilon dynamics suggests a mechanism for the establishment of leading-strand synthesis in the eukaryotic replisome. Proc. Natl Acad. Sci. USA 114, 4141–4146 (2017).
Ho, B., Baryshnikova, A. & Brown, G. W. Unification of protein abundance datasets yields a quantitative Saccharomyces cerevisiae proteome. Cell Syst. 6, 192–205 (2018).
Hirota, K. et al. In vivo evidence for translesion synthesis by the replicative DNA polymerase δ. Nucleic Acids Res. 44, 7242–7250 (2016).
Hirota, K. et al. The POLD3 subunit of DNA polymerase δ can promote translesion synthesis independently of DNA polymerase ζ. Nucleic Acids Res. 43, 1671–1683 (2015).
Tellier-Lebegue, C. et al. The translesion DNA polymerases Pol ζ and Rev1 are activated independently of PCNA ubiquitination upon UV radiation in mutants of DNA polymerase δ. PLoS Genet. 13, e1007119 (2017).
Pagès, V., Maria, S. R. S., Prakash, L. & Prakash, S. Role of DNA damage-induced replication checkpoint in promoting lesion bypass by translesion synthesis in yeast. Genes Dev. 23, 1438–1449 (2009).
Sabbioneda, S., Bortolomai, I., Giannattasio, M., Plevani, P. & Muzi-Falconi, M. Yeast Rev1 is cell cycle regulated, phosphorylated in response to DNA damage and its binding to chromosomes is dependent upon MEC1. DNA Repair 6, 121–127 (2007).
Zhao, L. & Washington, M. T. Translesion synthesis: insights into the selection and switching of DNA polymerases. Genes 8, 24 (2017).
Gallego-Sánchez, A., Andrés, S., Conde, F., San-Segundo, P. A. & Bueno, A. Reversal of PCNA ubiquitylation by Ubp10 in Saccharomyces cerevisiae. PLoS Genet. 8, e1002826 (2012).
Kubota, T., Katou, Y., Nakato, R., Shirahige, K. & Donaldson, A. D. Replication-coupled PCNA unloading by the Elg1 complex occurs genome-wide and requires Okazaki fragment ligation. Cell Rep. 12, 774–787 (2015).
Zhuang, Z. et al. Regulation of polymerase exchange between Polη and Polδ by monoubiquitination of PCNA and the movement of DNA polymerase holoenzyme. Proc. Natl Acad. Sci. USA 105, 5361–5366 (2008).
Washington, M. T., Johnson, R. E., Prakash, S. & Prakash, L. Fidelity and processivity of Saccharomyces cerevisiae DNA polymerase η. J. Biol. Chem. 274, 36835–36838 (1999).
Sharp, P. M. & Li, W.-H. The codon adaptation index—a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15, 1281–1295 (1987).
Frigola, J., Remus, D., Mehanna, A. & Diffley, J. F. X. ATPase-dependent quality control of DNA replication origin licensing. Nature 495, 339–343 (2013).
Coster, G., Frigola, J., Beuron, F., Morris, E. P. & Diffley, J. F. X. Origin licensing requires ATP binding and hydrolysis by the MCM replicative helicase. Mol. Cell 55, 666–677 (2014).
Acknowledgements
We thank J. Diffley for plasmids and yeast strains and J. Sale for critical reading of the manuscript. This work was supported by the Medical Research Council, as part of United Kingdom Research and Innovation (MRC grant no. MC_UP_1201/12 to J.T.P.Y). T.A.G. is supported by a Sir Henry Wellcome Postdoctoral Fellowship from the Wellcome Trust (213596/Z/18/Z).
Author information
Authors and Affiliations
Contributions
T.A.G. performed the experiments. T.A.G. and J.T.P.Y. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Beth Moorefield was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Extended data
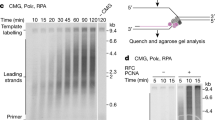
Extended Data Fig. 1 Pol η promotes TLS of lagging and leading-strand CPDs.
a, Purified Okazaki fragment processing and TLS proteins. b, Long exposure of the denaturing gel shown in Fig. 1b showing the diffuse ~1.7 kb stall product produced on the lagging-strand CPD template. c, Two-dimensional gel of the reaction performed in the absence of Pol η on the undamaged leading-strand template, shown in lane 1 of main text Fig. 1d. d, Two-dimensional gel of the reaction performed in the absence of Pol η on the leading-strand CPD template, shown in lane 7 of main text Fig. 1d. e, Two-dimensional gel of the reaction performed in the presence of 16 nM Pol η on the leading-strand CPD template, shown in lane 12 of main text Fig. 1d.
Extended Data Fig. 2 Leading-strand TLS occurs uncoupled from CMG.
a, Oligonucleotide competition assay performed in the absence or presence of Pol η. Reaction products were cleaved with SwaI to truncate stall products before resolution on a urea polyacrylamide gel. Addition of Pol η promotes extension of the stall product in the gap left behind from oligonucleotide-mediated recoupling. b, Reaction scheme for the pulse-chase experiment shown in (c). c, Pulse chase experiment on the leading-strand CPD template with 5 nM Pol η added 3 min into the pulse, at the start of the chase, or 10 min into the chase.
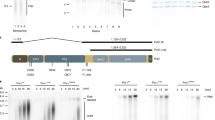
Extended Data Fig. 3 Pol δ, but not Pol ε, inhibits lagging and leading-strand TLS.
a, Pol δ titration into standard replication reactions on the undamaged leading-strand template containing Fen1 and Ligase. b, Denaturing gel of the reaction products from main text Fig. 3a. c, Standard replication reaction on the lagging-strand CPD template in the presence of 5 nM Pol η and increasing concentrations of Pol δ, as performed in Fig. 3a, but in the absence of Fen1 and Ligase. d, Pol δ titration into standard replication reactions on the undamaged leading-strand template. e, Reaction scheme for the pulse-chase experiment shown in (f). f, Pulse chase experiment on the leading-strand CPD template with 5 nM Pol η alone, or with 5 nM extra Pol ε, or Pol δ, added at the start of the chase.
Extended Data Fig. 4 Uncoupled replication forks display a recoupling defect in the absence of Pol δ.
a, Reaction scheme for the pulse-chase experiment shown in (b). b, Pulse chase experiment on the leading-strand CPD template in the absence of Pol δ and the absence or presence of 5 nM Pol η, added at the start of the chase. c, Two-dimensional gel of the 20 min time point shown in lane 6 of (b). d, Two-dimensional gel of the 20 min time point shown in lane 12 of (b).
Extended Data Fig. 5 PCNA monoubiquitination stimulates on the fly TLS.
a, Western blot of PCNA from standard 60 min replication reactions on the leading-strand CPD template, or undamaged equivalent, in the absence or presence of Fen1 and Ligase. All reactions contained ubiquitin, Uba1, and Rad6–Rad18 in addition to standard replication proteins. Denaturing gel of reaction products is shown below. b, Standard replication reaction time course on the undamaged template performed in the absence or presence of Rad6–Rad18, Uba1, and ubiquitin. c, Standard replication reaction time course on the leading-strand CPD template in the presence of 2.5 nM Pol η and 0.3 nM or 2.5 nM Pol δ. Samples were treated with BamHI and SwaI to generate bypass and stall products prior to resolution on the urea polyacrylamide gel. d, Denaturing gel of the reaction products from Fig. 5c. e, Replication reaction time course performed on the leading-strand CPD template in the absence or presence of Uba1 or Rad6–Rad18. Reactions contained 2.5 nM Pol η, 2.5 nM Pol δ, 1 μM ubiquitin, 5 nM Fen1, and 5 nM Ligase, in addition to standard replication proteins. Urea polyacrylamide gel samples were treated with BamHI and SwaI to generate quantifiable bypass and stall products. f, Quantification of the data in (e) showing the percentage of bypass in the absence or presence of uba1 or Rad6–18. g, Replication reaction time course performed on the leading-strand CPD template in the presence of PCNA monoubiquitination machinery (Rad6–Rad18, Uba1, and ubiquitin) and Pol η and the absence or presence of Pol δ.
Extended Data Fig. 6 Characterization of PCNAK164R.
a, Standard replication reaction time course performed on the undamaged template with either wild type PCNA or PCNAK164R. b, Standard replication reactions on the leading-strand CPD template containing increasing amounts of wild type PCNA or PCNAK164R. Reactions contained 5 nM Pol η. c, Standard replication reactions on the leading-strand CPD template in the absence or presence of 2.5 nM Pol δ and increasing amounts of wild type PCNA. Reactions contained 5 nM Pol η. d, Standard replication reaction time course on the undamaged leading-strand template with either wild type PCNA or PCNAK164R in the presence of Fen1 and Ligase.
Extended Data Fig. 7 PCNA monoubiquitination stimulates on the fly TLS in the absence of Fen1 and Ligase.
a, Standard replication reaction time course performed with wild-type PCNA in the absence or presence of Uba1. Reactions contained 10 nM Pol η, 10 nM Pol δ, 250 nM ubiquitin, and 200 nM Rad6–Rad18, in addition to standard replication proteins. Urea polyacrylamide gel samples were treated with BamHI and SwaI to generate quantifiable bypass and stall products. b, Quantification of the percentage of bypass in the absence or presence of Uba1 as performed in (b). Data are plotted as the means and s.e.m. of three independent experiments. c, Standard replication reaction time course performed with PCNAK164R in the absence or presence of Uba1. Reactions contained 10 nM Pol η, 10 nM Pol δ, 250 nM ubiquitin, and 200 nM Rad6–Rad18, in addition to standard replication proteins. Urea polyacrylamide gel samples were treated with BamHI and SwaI to generate quantifiable bypass and stall products. d, Quantification of the percentage of bypass in the absence or presence of Uba1 as performed in (d). Data are plotted as the means and s.e.m. of three independent experiments.
Extended Data Fig. 8 PCNA monoubiquitination promotes lagging-strand TLS.
a, Standard replication reaction time course on the lagging-strand CPD template in the presence of 2.5 nM Pol η and 0.3125 nM or 2.5 nM Pol δ. Samples were treated with BamHI and SwaI to generate bypass and stall products prior to resolution on the urea polyacrylamide gel. b, Denaturing gel of the reaction products from Fig. 6a.
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Source data
Source Data Fig. 1
Uncropped gels for Fig. 1.
Source Data Fig. 2
Uncropped gels for Fig. 2.
Source Data Fig. 3
Uncropped gels for Fig. 3.
Source Data Fig. 4
Uncropped gels for Fig. 4.
Source Data Fig. 5
Uncropped gels for Fig. 5.
Source Data Fig. 6
Uncropped gels for Fig. 6.
Rights and permissions
About this article
Cite this article
Guilliam, T.A., Yeeles, J.T.P. Reconstitution of translesion synthesis reveals a mechanism of eukaryotic DNA replication restart. Nat Struct Mol Biol 27, 450–460 (2020). https://doi.org/10.1038/s41594-020-0418-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-020-0418-4
This article is cited by
-
CST–polymerase α-primase solves a second telomere end-replication problem
Nature (2024)
-
DNA replication and replication stress response in the context of nuclear architecture
Chromosoma (2024)
-
Synergism between CMG helicase and leading strand DNA polymerase at replication fork
Nature Communications (2023)
-
Lagging strand gap suppression connects BRCA-mediated fork protection to nucleosome assembly through PCNA-dependent CAF-1 recycling
Nature Communications (2022)
-
Global landscape of replicative DNA polymerase usage in the human genome
Nature Communications (2022)